Feasibility and reproducibility of substituting oral contrast with water for duodenal volume delineation in patients undergoing pancreatic stereotactic body radiotherapy
Introduction
There is currently a major focus of investigation of stereotactic body radiotherapy (SBRT) for pancreatic neoplasms. However, resulting from the close anatomic pancreaticoduodenal relationship, SBRT planning and delivery are challenging vis-à-vis potential for duodenal toxicity (1,2). Additionally, owing to the relatively rapid development and institution of pancreatic SBRT, technical nuances of SBRT setup and simulation have been understudied and are largely institution-dependent (3).
Currently, per initial studies, four-dimensional simulation is most often preceded by fiducial placement and intake of oral contrast. However, these methods have several noteworthy shortcomings. First, rapid intake of an oral contrast bolus may not provide desired results, owing to expected versus observed transit time. If there is too short of a time differential between intake and simulation scan, the duodenum becomes over distended and substantially irreproducible between simulation and SBRT delivery (alternatively, contrast remains in the stomach). If the interval is too long, most contrast material goes through the duodenum and target delineation becomes difficult. Next, fiducial markers do not circumvent the need to add nontrivial margins around the target volume. There is often soft tissue distortion around the area from many extra-respiratory sources; thus, even with kilovoltage cone-beam image guidance, neither bony nor fiducial registration accurately provides a surrogate for the “true” target (4).
A major cause of this soft tissue misalignment is from the duodenum, as during simulation the duodenum can be artificially distended to some degree from oral contrast. During treatments, however (depending on the prandial status), the duodenum is more collapsed. This potentially leads to substantially higher doses delivered during treatment, owing to the sharp dose drop-off within each millimeter from the field. Potential solutions to this problem, including re-planning and gated treatment, are incompletely understood and are currently used based on physician preference only (5,6). We intent to discover an oral contrast that patient can use for daily radiation therapy.
Methods
This study examined 13 unresectable/borderline resectable pancreatic cancer patients simulated with water (January 2015 to August 2016) with comparison to 40 unresectable/borderline resectable patients treated on a prospective trial (NCT01068327) that utilized oral contrast. With the exception of the material ingested, all logistic elements and treatment planning was identical per institutional protocol.
Prior to simulation, all patients underwent fiducial marker implantation (two 2 mm x 5 mm VISICOIL gold seeds were implanted approximately 2 cm apart adjacent to the tumor). Simulation with a free-breathing CT and four-dimensional CT (4DCT), occurring at a minimum of 7 days after fiducial placement, was carried out using body fixation and immobilization devices (Medical Intelligence, Schwabmunchen, Germany). Intravenous contrast was given unless renal function precluded administration. The 13 patients that were evaluated for this report ingested 8 ounces of water, 15–20 min prior to simulation, similar to those that swallowed oral contrast.
The duodenum was defined as the duodenal bulb to the point the transverse duodenum crossed the left lateral border of the aorta; this (as well as contouring of other organs-at-risk) was performed in accordance with Radiation Therapy Oncology Group (RTOG) guidelines (7). Dose constraints used were per our institutional trial (NCT01068327) which were initially designed according to many sources, including previous studies (8-10), SBRT dose tolerance publications (11), the RTOG 0631 protocol (12), and previous dosimetric studies of SBRT for pancreatic cancer.
The gross tumor volume (defined as visible disease) was contoured using either Eclipse or BrainLab software, with 5 mm expansion to form the planning target volume (PTV). No prophylactic radiation to the regional lymphatic drainage area, similar to published work (8-10). The prescribed dose was required to cover 95% of the PTV at minimum.
The 13 patients receiving water during simulation were instructed to take the same amount at the same time prior to each SBRT session. Daily image guidance with kilovoltage cone-beam CT (CBCT) was performed, and owing to the quality of the imaging, re-contouring of the duodenum on each pre-treatment CBCT was not possible. Each treatment was performed using the Varian TrueBeam linear accelerator with a board-certified radiation oncologist supervising each session.
SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was utilized for statistics, and P<0.05 was considered statistically significant. Comparisons of the duodenal volume were performed by the Mann-Whitney U test.
Results
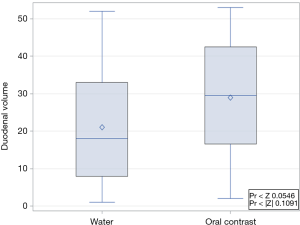
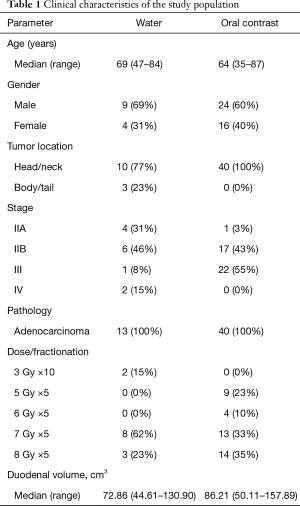
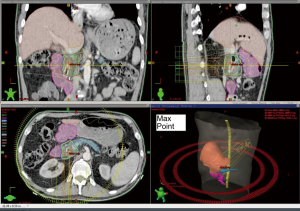
Clinical characteristics of both the populations receiving water and oral contrast are displayed in Table 1. In all patients, in the absence of a quantitative measure, the duodenum was able to be subjectively identified on the simulation CT (Figure 1). In the water group, the median volumes of duodenum and stomach were 72.86 cm3 (range, 44.51–130.90 cm3) and 350.27 cm3 (range, 66.37–1,314.19 cm3), respectively. In the oral contrast group, median volumes were 86.21 cm3 (range, 50.11–157.89 cm3) and 341.03 cm3 (range, 134.65–1,134.88 cm3). There were no significant differences between groups in median duodenal and gastric volumes (Figure 2, P=0.115 for duodenum and 0.813 for stomach). All patients were able to drink the same amount of water 15–20 min prior to each fraction of SBRT to keep the duodenum volume subjectively the same as it was on the simulation CT scan.
Full table
Discussion
SBRT is an emerging treatment option for pancreatic cancer, used primarily for locally advanced (unresectable) diseases, as it can potentially provide local tumor control without significant disadvantages for patients’ quality of life (13). Feasibility and efficacy has been shown in neoadjuvant settings (14), elderly patients (15), those with many comorbidities (16), and re-irradiation cases (17).
Our institution has employed a novel strategy for these patients that has resulted in high reproducibility and ultimately, low observed duodenal toxicities. In patients both on- and off-protocol, we have observed no grade 2+ duodenal toxicities which have been attributed to SBRT thus far in utilization of this strategy. The commonly duodenal-associated toxicities were grade-1 dyspepsia, poor appetite, nausea, and abdominal discomfort/pain. Recently, we performed a secondary dosimetric analysis to examine any possible associations among dosimetric parameters, histologic damage to the duodenum, and clinical toxicities in patients who had pancreaticoduodenectomy from our institutional phase I neoadjuvant SBRT trial. Our study showed that duodenal histologic damage but not the clinical toxicities correlate with the mean duodenal dose, V20-V35, and the PTV mean/maximum doses. In this cohort, four grade-2 and one grade-3 acute toxicities were observed (18).
Using water, target, duodenal, and gastric volume delineation is comparably similar to that with oral contrast, as the hypodense nature of water and the higher-density duodenal wall provide a high-quality barometer for delineating the clinical borders of the duodenum and stomach. Moreover, this setup is associated with high reproducibility for each treatment, although it is an admittedly a subjective measure. In addition, due to tumor motion with an average peak-to-peak amplitude of 15 mm in the craniocaudal direction, 5 mm in the anteroposterior direction and 3 mm in the lateral direction has been reported by Heerkens et al. (19), gating delivery as well as intestinal filling with water with the presented drinking protocol may reduce variability. Lastly, we performed dosimetric analysis and found that the median duodenal max and mean doses in the water group were significantly smaller than those in the contrast group (max: 31 vs. 37 Gy, P=0.005; mean: 12 vs. 17 Gy, P=0.009). The median gastric max dose in the water group was significantly smaller than that in the contrast group (25 vs. 33 Gy, P=0.017). However, there was no difference between groups in the stomach median mean dose (4 vs. 6 Gy, P=0.750). The superior dosimetric profile for the water group can be explained as improved planning technique as patients in the water group are planned more recently. Furthermore, it is important to consider that the “in vivo dosimetry” during actual treatments may result in an even more superior profile for the water group. This is due to the fact that oral contrast is typically not ingested prior to each treatment and the duodenum is presumably collapsed during SBRT delivery, as opposed to daily pre-SBRT ingestion of water.
There are limitations to our study. First, the retrospective nature and low sample sizes can never exclude selection bias; but this issue will likely not be studied prospectively; and the group receiving water were consecutive patients with the comparator arm a group of prospectively-collected patients. Second, the limitations of the quality of kilovoltage CBCT in providing accurate estimates (of what subjectively constituted a “similar-looking duodenum” as the simulation CT) is clearly apparent. Rather, it should be prominently mentioned that the goal of this communication is to put forth a novel technique that should be “subjectively corroborated” by other investigators and utilized in their own clinical practices in order to individually assess whether this method is of utility for their patients.
In summary, to our knowledge, this method has not been described before and is used rarely (if at all) at the present time. Nevertheless, we encourage further use and study of this method for the known technical challenges posed by pancreatic SBRT.
Acknowledgements
Funding: This work is supported by DHHS/NIH/NCI SPORE grant in pancreatic cancer (P50 CA127297-06A1).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: The study was approved by University of Nebraska Medical Center Institutional Review Board (IRB# 646-16-EP) and informed consent was waived by the IRB because it is a retrospective study.
References
- Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2008;72:678-86. [Crossref] [PubMed]
- Verma V, Li J, Lin C. Neoadjuvant therapy for pancreatic cancer: systematic review of postoperative morbidity, mortality, and complications. Am J Clin Oncol 2016;39:302-13. [Crossref] [PubMed]
- Huguet F, Goodman KA, Azria D, et al. Radiotherapy technical considerations in the management of locally advanced pancreatic cancer: American-French consensus recommendations. Int J Radiat Oncol Biol Phys 2012;83:1355-64. [Crossref] [PubMed]
- Yang W, Fraass BA, Reznik R, et al. Adequacy of inhale/exhale breathhold CT based ITV margins and image-guided registration for free-breathing pancreas and liver SBRT. Radiat Oncol 2014;9:11. [Crossref] [PubMed]
- Taniguchi CM, Murphy JD, Eclov N, et al. Dosimetric analysis of organs at risk during expiratory gating in stereotactic body radiation therapy for pancreatic cancer. Int J Radiat Oncol Biol Phys 2013;85:1090-5. [Crossref] [PubMed]
- Li Y, Hoisak JD, Li N, et al. Dosimetric benefit of adaptive re-planning in pancreatic cancer stereotactic body radiotherapy. Med Dosim 2015;40:318-24. [Crossref] [PubMed]
- Radiation Therapy Oncology Group. Upper abdominal normal organ contouring consensus guidelines. Accessed August 8, 2015. Available online: https://www.rtog.org/CoreLab/ContouringAtlases/UpperAbdominalNormalOrganContouringConsensusGuidelines.aspx
- Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009;115:665-72. [Crossref] [PubMed]
- Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;81:181-8. [Crossref] [PubMed]
- Gurka MK, Collins SP, Slack R, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety. Radiat Oncol 2013;8:44. [Crossref] [PubMed]
- Grimm J, LaCouture T, Croce R, et al. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys 2011;12:3368. [Crossref] [PubMed]
- Radiation Therapy Oncology Group. RTOG 0631 Protocol Information. Accessed August 8, 2015. Available online: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0631
- Kishan A, Lee P. Having Your Cake and Eating It Too: Combining SBRT and Multi-agent Chemotherapy in Locally Advanced Pancreatic Cancer. Cureus 2016;8:e686. [PubMed]
- Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013;86:516-22. [Crossref] [PubMed]
- Kim CH, Ling DC, Wegner RE, et al. Stereotactic body radiotherapy in the treatment of pancreatic adenocarcinoma in elderly patients. Radiat Oncol 2013;8:240. [Crossref] [PubMed]
- Yechieli RL, Robbins JR, Mahan M, et al. Stereotactic Body Radiotherapy for Elderly Patients With Medically Inoperable Pancreatic Cancer. Am J Clin Oncol 2017;40:22-6. [Crossref] [PubMed]
- Lominska CE, Unger K, Nasr NM, et al. Stereotactic body radiation therapy for reirradiation of localized adenocarcinoma of the pancreas. Radiat Oncol 2012;7:74. [Crossref] [PubMed]
- Verma V, Lazenby AJ, Zheng D, et al. Dosimetric parameters correlate with duodenal histopathologic damage after stereotactic body radiotherapy for pancreatic cancer: Secondary analysis of a prospective clinical trial. Radiother Oncol 2017;122:464-9. [Crossref] [PubMed]
- Heerkens HD, van Vulpen M, van den Berg CA, et al. MRI-based tumor motion characterization and gating schemes for radiation therapy of pancreatic cancer. Radiother Oncol 2014;111:252-7. [Crossref] [PubMed]