Primary pancreatic lymphoma: what we need to know
Introduction
Non-hodgkin’s lymphoma (NHL) is a group of malignant lymphoids which can be seen at any age. It is often determined with enlarged lymph nodes, fever, and weight loss. NHL commonly affects extranodal organs while the involvement of pancreas is primarily rare and can be with or without the involvement of lymph nodes around the pancreas (1). Differentiation of primary pancreatic lymphoma (PPL) and adenocarcinoma is important due to different treatment and better prognosis; however, the symptoms of PPL are not specific and can mimic adenocarcinoma. PPL can often be seen as a mass larger than 5 cm in the pancreas while unlike adenocarcinoma, no vascular involvement is commonly observed. The definitive diagnosis of PPL is impossible based on imaging and it requires pathological examination. CT-guided biopsy and laparotomy have been proposed for obtaining tissue samples. Fine needle aspiration (FNA) through endoscopic ultrasound (EUS) (EUS-FNA) has been a good method to obtain tissue samples through endoscopic ultrasound in recent years (2).
Epidemiology
PPL consists of approximately 1% of all extranodal lymphomas and 5% of all pancreatic masses (1). PPLs are more common in males (58%) and are usually seen in the 5th or 6th decades of life.
The most histological type of PPL is diffuse large B cell lymphoma which forms 80% of all cases, however, other histologic types may rarely be seen (3).
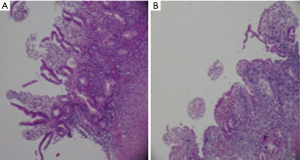
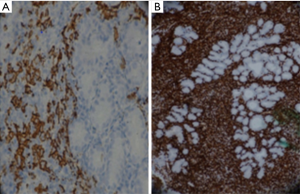
Rad et al. (4) reported on patient with pancreatic mass and icter who had low grade B cell lymphoma in the histological examination (Figures 1 and 2).
Clinical symptoms
Clinical symptoms of PPL are not specific for the disease and include epigastric pain, abdominal mass, weight loss, jaundice, nausea, vomiting, diarrhea, pancreatitis, and intestinal obstruction (5,6).
PPLs rarely represent B symptoms which are often seen in other lymphoma and include fever, night sweats, and weight loss. The most symptoms of PPLs are with vague abdominal discomforts such as dyspepsia, pain, nausea, vomiting, feeling of fullness, and body weight loss (7).
Dawson et al. proposed five criteria for the diagnosis of PPL (8):
- Lack of peripheral lymphadenopathy;
- Lack of involvement of mediastinal lymph node;
- Counting normal peripheral white blood cells;
- Pancreatic mass specified in surgery with the involvement of lymph nodes confined to the pancreas;
- Lack of involvement of liver or spleen.
Imaging techniques
Transabdominal ultrasound (TUS)
TUS is a technique dependent on the accuracy of operator and has a low precision to see small masses in the head of the pancreas. TUS can indicate dilatation and obstruction of the bile ducts and liver metastases (9). Using new methods of ultrasound such as Color-power Doppler us, 3 dimensional (3D) US, and harmonic imaging, contrast-enhanced us, the diagnosis of pancreatic masses has significantly enhanced (10). Using contrast enhanced us, vascular involvement can be investigated in the pancreas masses that can help the differentiation of pancreatic masses (11).
Abdominal CT scan
To examine pancreas masses and liver metastases, CT scan which is an accessible and noninvasive method can be used and based on the results of CT scan, decisions can be made on sampling to prove the diagnosis. The quality of CT scan images has been improved using multiple detector CT which creates images with high resolution 3-D imaging and multiplanar ones. Rapid injection of iodine-containing contrast and images that are immediately taken after injection are among the methods to increase the sensitivity of CT in the evaluation of pancreatic masses (11). CT sensitivity is low for lesions less than 2 cm (12-14).
CT scan guide can be used for the biopsy of pancreatic lesions with the sensitivity about 95% (15,16). Radiological results from the previous studies in which the secondary lymphoma of the pancreas was similar to primary lymphoma include nodular, diffuse and multinodular (6,17,18). Most masses have been recognized through intravenous injection by CT scan well and sometimes are bulky and infiltrated. Lesions with the homogeneous low attenuation are along with a slight enhancement of pancreatic parenchyma (5).
Pancreatic lymphoma with a lower incidence may show the symptoms of acute pancreatitis on CT scan which appears as diffuse pancreatic enlargement while the results of typical pancreatitis in CT including inflammation around the pancreas or fat stranding do not exist; otherwise, they can be seen usually minimal. Fluid accumulation around the pancreas, pancreatic fat necrosis and rupture of the pancreatic duct are not seen in lymphoma. Vascular involvement by tumor can rarely be seen in lymphoma (5). Although pressure may be observed on vessels due to the effect of the mass, there are no changes resulting from the conflict involvement of tumor including a change in caliber and irregularity (19).
MRI
MRI is a good diagnostic method for pancreatic masses and is superior to CT in terms of tissue contrast. Pancreatic lymphoma can be seen as homogeneous, low signal intensity and focal nodular in T1W1 images and high or low signal intensity in T2W1 images. In DCE-MRI, low enhanced area surrounded by parenchyma is usually observed (5,18). Unlike CT, the lesions of pancreatic lymphoma are mildly heterogeneous in MRI, especially in T2W images. Tumors of Islet cell have more hyper intensity than lymphoma in T2w images.
Whole body DW1 had formerly a significant role in diagnosing and staging the disease in lymphoma patients. By receiving intravenous gadolinium contrast, lymphoma is homogeneously enhanced in a less degree than normal parenchyma. A number of lymphomas appear softly inhomogeneous in MRI. Due to the desmoplastic content, the pancreas adenocarcinoma is less enhanced and after receiving gadolinium, they are typically inhomogeneous. Due to the difference in the treatment and prognosis of pancreatic lymphoma, its differentiation from similar lesions such as pancreatic autoimmune and autoimmune pancreatitis seems to be of great significance (20).
Merkle et al. (5) offered the following criteria for distinguishing lymphoma from pancreatic autoimmune:
- Huge homogeneous mass without cystic-necrosis lesions or MPD involvement;
- Adjacent arteries involvement without obstruction;
- If there were lymphadenopathy under the left renal vein, it would be limited to the area around the pancreas.
However, despite the above criteria, CT-guided biopsy or EUS FNA is important for an accurate diagnosis in most cases.
EUS
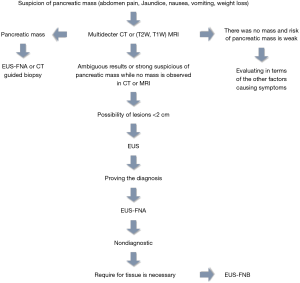
Although EUS is dependent on operator, it is more sensitive than CT or MRI to detect suspicious pancreatic lesions or lesions less than 2 cm on CT if it is done by an experienced person (12,14,21-23). Tissue sampling by EUS can be done in two ways: either by EUS guided FNA (EUS-FNA) or EUS guided fine-needle core biopsy (EUS-FNB). The sensitivity of EUSFNA is 95% and its specificity is 100% (24-27). It is the preferred method for sampling the pancreatic tissue, especially when the biopsy results of other methods are negative or ambiguous for malignancy (28,29). Although EUSFNA along with cytopathology are usually suitable for the diagnosis of adenocarcinoma and neuroendocrine tumors, it may not well obtain the tissue required for the full tissue examination in order for the diagnosis of lymphoma-pancreatitis cancer and autoimmune (30,31). EUS-FNB is not superior to EUS-FNA for the diagnosis of pancreatic masses. However, it is used in the events where EUS-FNA does not help make a diagnosis and tissue samples are needed (32-37). Due to the rigidity of the needle and angle of the endoscope for sampling, obtaining a sample of pancreatic masses by EUS-FNB is hard (38). We have designed an algorithm for evaluating patients with suspected pancreatic mass (Figure 3).
The complications of EUS-guided sampling of the masses of pancreas include hemorrhage (0.5–2% of the cases) (24,26,27,39,40) and tumor seeding in other parts of the abdomen whose risk is very low (41-43). The study conducted by Khashab et al. (44) indicated that EUS-FNA by flow cytometry was preferred to EUSFNA without flow cytometry in the assessment of 16 patients suspected to primary pancreas lymphoma.
In another study, Ramesh et al. (45) showed that from 2,397 patients undergoing EUS-FNA due to the solid mass of pancreas, 12 patients had PPL and the mean of the largest diameter of the mass was 47.5 mm (SD =21) and over 80% of the cases were in the head of pancreas. Echo image was heterogeneous in 75% of cases, while the rest were hypoechoic.
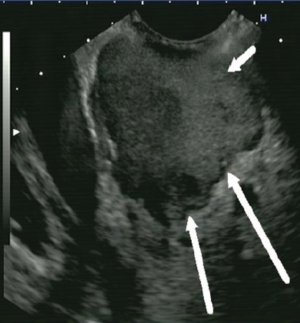
Abedi et al. (46) reported on a case report who was a 38-year-old man with the history of smoking, Intravenous Drug Using (IVDU), and hepatitis B and C and was admitted due to the nausea, vomiting, RUQ pain, and epigastric pain. EUSFNA performed for the patient and indicated a mixed echo mass in the pancreatic head invading the portal vein and SMA (superior mesenteric artery) and SMV (Figures 4,5). In the pathobiological examination of tumor tissue, small round cells which were a sign of lymph proliferative disorder was seen and the examination of IHC proved the diagnosis of lymphoma.
Tumor markers
Serum carbohydrate antigen 9-19 (CA19-9) level increases rarely in patients with PPL (47). In contrast to patients with adenocarcinoma which is high in 80% of cases, CA19-9 is sometimes increased modestly in patients with PPL due to biliary obstruction (48). LDH serum can enhance in Lymphoproliferative disorders such as NHL; however, an increase in LDH is not required for the diagnosis of PPL (49).
Differential diagnoses
PPL is a rare disorder which can be present as a focal or diffuse mass and imitate the properties of common pancreas tumors such as adenocarcinoma or inflammatory process such as pancreatitis (47,50,51).
Adenocarcinoma: pancreatic adenocarcinoma includes most pancreatic tumors; however, approximately 10–15% of masses are for other reasons such as cystic neoplasms and neuroendocrine tumors (52).
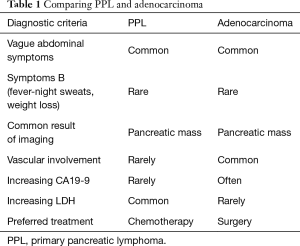
Differentiation PPL from adenocarcinoma is important since PPL has better prognosis even in advanced cases and can potentially be better treated. Due to the rarity of PPL and nonspecific clinical symptoms and imaging, differentiation of PPL from adenocarcinoma is very difficult without cytopathology. The tissue sampling can be performed through FNA under CT, EUS guides or sampling within surgery. Sampling by EUS or CT is preferred because it avoids unnecessary surgery and complications (7). Table 1 shows a comparison of PPL and adenocarcinoma.
Full table
Pancreatic neuroendocrine tumors: most pancreatic neuroendocrine tumors are sporadic; however, they may be associated with inherited genetic syndromes such as Multiple Endocrine Neoplasia (MEN) type 1 and 2.
They are without function in 45–91% of cases. Most tumors of functional neuroendocrine are insulinoma and then, glucagonoma, gastrinoma (Zollinger-Ellison syndrome), and somatostatinoma (53).
EUS-FNA has a sensitivity of over 90% for the diagnosis (54,55) and is useful to obtain tissue samples to examine the expression of Ki-67 so that prognostic factor is in the pancreatic endocrine tumors of the pancreas (53).
Inflammatory process: PPL is a controversial diagnosis as its radiological and clinical results are common to other pancreatic disorders such as AIP (an inflammatory disease of the pancreas which is usually determined by painless jaundice of pancreatic mass or enlargement and response to corticosteroids).
AIP diagnostic criteria include typical imaging findings of CT or MRI dynamics, increasing the level of IgG4, involvement of other organs (renal mass, tubulointerstitial nephritis, sclerosing cholangitis, retroperitoneal fibrosis, and submandibular mass), response to steroids and if available, examination of histology and immunostaining. Type 1 AIP is diagnosed without histological confirmation. In the cases that CT or MRI findings are typical for AIP and there are serum elevation of IgG4 or the involvement of other organs and in cases where diagnosis is not decisive based on other examinations, histological cytology is need (56).
Anderloni et al. (57) reported a case of primary pancreas lymphoma in a young woman with jaundice, fever, and abdominal pain that the patient’s symptoms were Similar to autoimmune pancreatitis. Clinical examination by CT scan of abdomen and an endoscopy of upper GI showed a large duodenal mass. Endoscopic biopsy was done and the results were consistent with the primary lymphoma of the pancreas.
Abdi et al. (46) also reported a case of PPL presented with acute pancreatitis (Nausea, vomiting, RUQ and epigastric pain, high level of amylase 480 U/L, and lipase 326 U/L).
Metastatic diseases: metastasis to the pancreas is rare and no area of the pancreas is preferred (58). Most metastasis to pancreas is from renal cell carcinoma; however, metastasis has also been seen from other tumors including breast, lung, and colorectal tumors. There is usually a long delay between initial diagnosis of tumor and the presence of metastatic pancreas and multiple metastases may exist at the time of diagnosis (59). Metastasis to pancreas can result in the obstruction of bile duct or pancreatic duct, pain, and pancreatitis (60,61). The history of previous malignancy raises the possibility of metastatic lesion for pancreas. Thus, the examination for immunostains or core biopsy should be considered. EUSFNA by 22 gauge needle with immunostaining is a good diagnostic method in patients with unusual neuroendocrine or metastatic lesions (61).
Treatment
Treatment and prognosis of PPL depends on the stage and grade of the disease. Most PPLs are of diffuse large B cell type. According to recent reports, there have been a prolong remission in several cases of PPL with chemotherapy (62). The first-line of chemotherapy regimen is with prednisolone-vincristine-doxorubicin-cyclophosphamide (6,49). In some case of diffuse large B cell with positive CD20, rituximab is added to the above regimen and increases the rate of remission (3). The combination of radiotherapy and chemotherapy has been used in some cases; however, its effect has not been proven yet (63). In PPL, surgery is difficult because the tumor is large and along with the normal histology of other parts of pancreas and there is a high risk of pancreatic fistula after surgery as well (64).
Conclusions
PPL are rare lesions but potentially treatable by chemotherapy and have symptoms similar to other pancreatic malignancies and inflammatory lesions. Correct diagnosis of PPL is important to avoid unnecessary surgery. EUSFNA is a preferred method to obtain tissue sample for the diagnosis of PPL.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zucca E, Roggero E, Bertoni F, et al. Primary extranodal non-Hodgkin's lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol 1997;8:727-37. [Crossref] [PubMed]
- Fukita Y, Asaki T, Adachi S, et al. Non-Hodgkin Lymphoma Mimicking Pancreatic Adenocarcinoma and Peritoneal Carcinomatosis. J Clin Oncol 2013;31:e373-6. [Crossref] [PubMed]
- Alexander RE, Nakeeb A, Sandrasegaran K, et al. Primary pancreatic follicle center-derived lymphoma masquerading as carcinoma. Gastroenterol Hepatol (N Y) 2011;7:834-8. [PubMed]
- Rad N, Heidarnezhad A, Soheili S, et al. A Man with Pancreatic Head Mass Lesion on Endoscopic Ultrasound and Granuloma on Cytopathology. Case Rep Gastroenterol 2016;10:760-8. [Crossref] [PubMed]
- Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am J Roentgenol 2000;174:671-5. [Crossref] [PubMed]
- Saif MW. Primary pancreatic lymphomas. JOP 2006;7:262-73. [PubMed]
- Li Z, Zhang S, Vasdani N, et al. Clues for diagnosing primary pancreatic lymphoma. Case Rep Gastroenterol 2012;6:438-45. [Crossref] [PubMed]
- Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg 1961;49:80-9. [Crossref] [PubMed]
- Freeny PC. Pancreatic carcinoma: what is the best imaging test? Pancreatology 2001;1:604-9. [Crossref] [PubMed]
- Hirooka Y, Goto H, Ito A, et al. Recent advances in US diagnosis of pancreatic cancer. Hepatogastroenterology 2001;48:916-22. [PubMed]
- Takeda K, Goto H, Hirooka Y, et al. Contrast-enhanced transabdominal ultrasonography in the diagnosis of pancreatic mass lesions. Acta Radiol 2003;44:103-6. [Crossref] [PubMed]
- Bronstein YL, Loyer EM, Kaur H, et al. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol 2004;182:619-23. [Crossref] [PubMed]
- Saisho H, Yamaguchi T. Diagnostic imaging for pancreatic cancer: computed tomography, magnetic resonance imaging, and positron emission tomography. Pancreas 2004;28:273-8. [Crossref] [PubMed]
- Sahani DV, Bonaffini PA, Catalano OA, et al. State-of-the-art PET/CT of the pancreas: current role and emerging indications. Radiographics 2012;32:1133-58. [Crossref] [PubMed]
- Erturk SM, Mortele KJ, Tuncali K, et al. Fine-needle aspiration biopsy of solid pancreatic masses: comparison of CT and endoscopic sonography guidance. AJR Am J Roentgenol 2006;187:1531-5. [Crossref] [PubMed]
- Paulsen SD, Nghiem HV, Negussie E, et al. Evaluation of imagingguided core biopsy of pancreatic masses. AJR Am J Roentgenol 2006;187:769-72. [Crossref] [PubMed]
- Cappell MS, Yao F, Cho KC, et al. Lymphoma predominantly involving the pancreas. Dig Dis Sci 1989;34:942-7. [Crossref] [PubMed]
- Ishigami K, Tajima T, Nishie A, et al. MRI findings of pancreatic lymphoma and autoimmune pancreatitis: a comparative study. Eur J Radiol 2010;74:e22-8. [Crossref] [PubMed]
- Lui PC, Wong GK, Poon WS, et al. Intravascular lymphomatosis. J Clin Pathol 2003;56:468-70. [Crossref] [PubMed]
- Fujinaga Y, Lall C, Patel A, et al. MR features of primary and secondary malignant lymphoma of the pancreas: a pictorial review. Insights Imaging 2013;4:321-9. [Crossref] [PubMed]
- Müller MF, Meyenberger C, Bertschinger P, et al. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology 1994;190:745-51. [Crossref] [PubMed]
- DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med 2004;141:753-63. [Crossref] [PubMed]
- Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc 1999;50:786-91. [Crossref] [PubMed]
- Lai R, Stanley MW, Bardales R, et al. Endoscopic ultrasound-guided pancreatic duct aspiration: diagnostic yield and safety. Endoscopy 2002;34:715-20. [Crossref] [PubMed]
- Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc 2005;62:728-36. [Crossref] [PubMed]
- Eloubeidi MA, Jhala D, Chhieng DC, et al. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer 2003;99:285-92. [Crossref] [PubMed]
- Eloubeidi MA, Chen VK, Eltoum IA, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol 2003;98:2663-8. [PubMed]
- Gress F, Gottlieb K, Sherman S, et al. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med 2001;134:459-64. [Crossref] [PubMed]
- Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol 2002;97:1386-91. [Crossref] [PubMed]
- Thomas T, Kaye PV, Ragunath K, et al. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: a large tertiary referral center experience. Am J Gastroenterol 2009;104:584-91. [Crossref] [PubMed]
- Levy MJ. Endoscopic ultrasound-guided trucut biopsy of the pancreas: prospects and problems. Pancreatology 2007;7:163-6. [Crossref] [PubMed]
- Levy MJ, Reddy RP, Wiersema MJ, et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc 2005;61:467-72. [Crossref] [PubMed]
- Shah SM, Ribeiro A, Levi J, et al. EUS-guided fine needle aspiration with and without trucut biopsy of pancreatic masses. JOP 2008;9:422-30. [PubMed]
- Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy 2004;36:397-401. [Crossref] [PubMed]
- Aithal GP, Anagnostopoulos GK, Tam W, et al. EUS-guided tissue sampling: comparison of "dual sampling" (Trucut biopsy plus FNA) with "sequential sampling" (Trucut biopsy and then FNA as required). Endoscopy 2007;39:725-30. [Crossref] [PubMed]
- Larghi A, Verna EC, Stavropoulos SN, et al. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc 2004;59:185-90. [Crossref] [PubMed]
- Mizuno N, Bhatia V, Hosoda W, et al. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol 2009;44:742-50. [Crossref] [PubMed]
- Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc 2012;76:336-43. [Crossref] [PubMed]
- Eloubeidi MA, Tamhane A, Varadarajulu S, et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 2006;63:622-9. [Crossref] [PubMed]
- Eloubeidi MA, Gress FG, Savides TJ, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc 2004;60:385-9. [Crossref] [PubMed]
- Paquin SC, Gariépy G, Lepanto L, et al. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc 2005;61:610-1. [Crossref] [PubMed]
- Chong A, Venugopal K, Segarajasingam D, et al. Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc 2011;74:933-5. [Crossref] [PubMed]
- Ahmed K, Sussman JJ, Wang J, et al. A case of EUS-guided FNA-related pancreatic cancer metastasis to the stomach. Gastrointest Endosc 2011;74:231-3. [Crossref] [PubMed]
- Khashab M, Mokadem M, DeWitt J, et al. Endoscopic ultrasound-guided fine-needle aspiration with or without flow cytometry for the diagnosis of primary pancreatic lymphoma - a case series. Endoscopy 2010;42:228-31. [Crossref] [PubMed]
- Ramesh J, Hebert-Magee S, Kim H, et al. Frequency of occurrence and characteristics of primary pancreatic lymphoma during endoscopic ultrasound guided fine needle aspiration: a retrospective study. Dig Liver Dis 2014;46:470-3. [Crossref] [PubMed]
- Abedi SH, Ahmadzadeh A, Nikmanesh A, et al. The role of endoscopic ultrasound in primary pancreatic lymphoma presented with acute pancreatitis: a case report. JOP 2014;15:493-6. [PubMed]
- Lin H, Li SD, Hu XG, et al. Primary pancreatic lymphoma: report of six cases. World J Gastroenterol 2006;12:5064-7. [Crossref] [PubMed]
- Stocken DD, Hassan AB, Altman DG, et al. Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer 2008;99:883-93. [Crossref] [PubMed]
- Du X, Zhao Y, Zhang T, et al. Primary pancreatic lymphoma: a clinical quandary of diagnosis and treatment. Pancreas 2011;40:30-6. [Crossref] [PubMed]
- Kondo T, Hayakawa T, Shibata T, et al. Pancreatic involvement by lymphoma simulates pancreatic carcinoma. J Clin Gastroenterol 1989;11:594-6. [PubMed]
- Sheth S, Fishman EK. Imaging of uncommon tumors of the pancreas. Radiol Clin North Am 2002;40:1273-87. [Crossref] [PubMed]
- Imaoka H, Yamao K, Bhatia V, et al. Rare pancreatic neoplasms: the utility of endoscopic ultrasound-guided fine-needle aspiration-a large single center study. J Gastroenterol 2009;44:146-53. [Crossref] [PubMed]
- Kulke MH, Benson AB, Bergsland E, et al. Neuroendocrine tumors. J Natl Compr Canc Netw 2012;10:724-64. [Crossref] [PubMed]
- Larghi A, Capurso G, Carnuccio A, et al. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: a prospective study. Gastrointest Endosc 2012;76:570-7. [Crossref] [PubMed]
- Unno J, Kanno A, Masamune A, et al. The usefulness of endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic neuroendocrine tumors based on the World Health Organization classification. Scand J Gastroenterol 2014;49:1367-74. [PubMed]
- Kamisawa T, Chari ST, Lerch MM, et al. Recent advances in autoimmune pancreatitis: type 1 and type 2. Gut 2013;62:1373-80. [Crossref] [PubMed]
- Anderloni A, Genco C, Ballarè M, et al. A case of primary pancreatic non-Hodgkin B-cell lymphoma mimicking autoimmune pancreatitis. J Gastrointestin Liver Dis 2015;24:245-8. [PubMed]
- Minni F, Casadei R, Perenze B, et al. Pancreatic metastases: observations of three cases and review of the literature. Pancreatology 2004;4:509-20. [Crossref] [PubMed]
- Baron TH. Endoscopic US for metastases to the pancreas: chasing the satellites. Gastrointest Endosc 2005;61:697-9. [Crossref] [PubMed]
- Jarufe N, McMaster P, Mayer AD, et al. Surgical treatment of metastases to the pancreas. Surgeon 2005;3:79-83. [Crossref] [PubMed]
- Sperti C, Pasquali C, Liessi G, et al. Pancreatic resection for metastatic tumors to the pancreas. J Surg Oncol 2003;83:161-6. [Crossref] [PubMed]
- Eloubeidi MA, Tamhane AR, Buxbaum JL. Unusual, metastatic, or neuroendocrine tumor of the pancreas: a diagnosis with endoscopic ultrasound-guided fine-needle aspiration and immunohistochemistry. Saudi J Gastroenterol 2012;18:99-105. [Crossref] [PubMed]
- Serin KR, Güven K, Ozden I, et al. Curative Chemoradiotherapy of Primary Pancreatic Lymphoma with Vertebral Metastasis: Palliation of Persistent Biliary Stricture by Roux-en-Y Hepaticojejunostomy. Case Rep Gastroenterol 2011;5:642-7. [Crossref] [PubMed]
- Koniaris LG, Lillemoe KD, Yeo CJ, et al. Is there a role for surgical resection in the treatment of early-stage pancreatic lymphoma? J Am Coll Surg 2000;190:319-30. [Crossref] [PubMed]