The role of neoadjuvant radiotherapy for locally-advanced rectal cancer with resectable synchronous metastasis
Introduction
Rectal cancer affects nearly 40,000 new patients each year in the United States (1). The treatment algorithm for locally-advanced rectal cancer without metastasis is well established. Currently the standard includes the delivery of neoadjuvant chemoradiation followed by total mesorectal excision. Treating rectal cancer patients without radiotherapy is currently being studied (2,3) as the use of chemotherapy in the adjuvant setting has become more common. Therefore, this series attempts to confirm the benefits of radiation therapy in the setting of resectable stage IV disease.
Approximately 20% to 30% of newly diagnosed rectal cancer patients present with synchronous metastasis (4). In the setting of widespread metastatic disease, chemotherapy is standard of care, while radiotherapy and/or surgery are only used for palliation. The value of radiotherapy is less clear in patients who present with resectable metastatic disease involving the liver and/or lung. Historically, surgical resection of the primary tumor and resection/ablation of all known metastases achieve 5-year overall survival (OS) rates as high as 71% (5-8).
Proper total mesorectal excision combined with neoadjuvant radiotherapy is known to reduced local recurrence (LR) rates significantly for patients with non-metastatic locally-advanced rectal cancer (9). However, its role and timing is less understood in patients presenting with synchronous metastasis. Modern retrospective series have reported pelvic recurrence rates of 12–34% in stage IV rectal cancer patients treated with curative surgery and adjuvant chemotherapy suggesting a potential role for neoadjuvant pelvic radiotherapy to optimize local disease control (4,10).
To date the lack of consensus regarding the role and timing of radiotherapy for patients with resectable stage 4 rectal cancer treated with curative intent (11) demands further research. The objective of this study is to examine the value of neoadjuvant radiotherapy for patients who are undergoing curative surgery for locally-advanced rectal cancer with synchronous metastasis.
Methods
Patients
We performed a retrospective review of a prospectively maintained surgical registry of all consecutive adult patients (age 18 years or older) who underwent curative-intent resection of primary locally-advanced rectal cancer (T3, T4 and/or nodal involvement) with synchronous liver and/or lung metastasis at Mayo Clinic, Rochester, MN from January 1990 until December 2014. The institutional review board approved this retrospective study. Metastasectomy was performed either simultaneously with resection of the primary tumor or as a planned staged resection. Exclusion criteria were as follows: patients with primary tumor stage of T1N0 or T2N0, patients with metastasis to organs other than the liver or lung, patients who had palliative resection, patients who had non-surgical treatment of synchronous metastasis (e.g., radiofrequency ablation), patients who received postoperative radiotherapy, or absence of research authorization.
Data
Data collected included patients’ demographics comprising age, gender, body mass index (BMI), and American Society of Anesthesiologists (ASA) Physical Status Classification. Perioperative data collected include distance of the tumor from the anal verge, site of metastasis at initial presentation, TNM stage of the disease, preoperative CEA level, procedure performed, addition of diverting stoma, type of anastomosis, operative time, number of lymph nodes harvested, status of surgical margins, use of radiotherapy, and the use of neoadjuvant and/or adjuvant chemotherapy (excluding that given concurrent with radiotherapy).
The American Joint Committee for Cancer (AJCC) seventh edition was used for cancer staging (12). For those patients without neoadjuvant radiation or chemotherapy, we used the postoperative pathological stage. For patients who had neoadjuvant therapy, the higher of preoperative clinical vs. postoperative pathological stage was used to determine the treated stage.
All patients underwent complete history and physical examination, laboratory evaluation, colonoscopy and biopsy of the lesion. Staging of the tumor was done by endorectal ultrasound and/or dedicated pelvic MRI. Distant metastases were evaluated by CT scan and/or PET scan. Use of neoadjuvant radiotherapy was at the discretion of the treating colorectal surgeon, medical oncologist, and/or radiation oncologists. Long course chemoradiotherapy typically consisted of 50.4 Gy in 28 fractions of RT delivered over 5 ½ weeks with concurrent 5FU, followed by a 6–8-week break before surgery. Short course radiotherapy consisted of 25 Gy in 5 fractions of RT without concurrent chemotherapy, followed by surgery within 1 week. Intraoperative radiotherapy was utilized for patients with T4 tumors and/or concern for residual local disease after complete resection.
After discharge, Mayo Clinic colorectal surgeons and medical oncologists typically followed the patients in a standard routine adopted from the National Comprehensive Cancer Network guidelines including history and physical examination, regular colonoscopy/sigmoidoscopy and imaging with CT scan as well as serum CEA levels.
Outcomes measures
The primary outcomes are LR, distant metastasis, overall (OS) and disease-specific survival (DSS). A LR is defined as the presence of a histologically proven tumor in the pelvis within the field of surgery. Distant metastasis is defined as any recurrence outside the pelvis. The endpoints of the study were survival and the presence of recurrence during the most recent follow-up.
Statistical analysis
Continuous variables were summarized with median and interquartile range and compared with a Mann-Whitney test. Categorical variables were described as frequencies (percent) and compared with Fisher’s exact test. All time-to-event outcomes (LR, distant metastasis, or cancer death) were considered as time from the surgery. For the analysis for local or distant recurrence, death of any cause was treated as competing risk in Cox proportional hazard models and comparisons were made with the log rank test (13). Survival curves for neoadjuvant RT status were generated using the Kaplan-Meier method and compared with the log rank test. Survival was stratified for year of LR to account for long time frame of series. All models were univariate, due to the few number of events. A level of 0.05 was defined as statistically significant. The analyses were performed using the statistical software package R v.3.1.2 and IBM SPSS version 23.
Results
Patients
During the study period, 93 patients with locally-advanced rectal cancer and synchronous liver and/or lung metastasis underwent curative-intent surgery. Of those, 47 patients received neoadjuvant radiotherapy (RT group) and 46 patients did not (no RT group). Of the patients who received neoadjuvant RT, 35 patients (75%) had long-course chemo-RT (median dose 50.4 Gy, range 30–55.8 Gy) whereas 12 patients (25.5%) had short-course RT (median dose 25 Gy, range 20–25 Gy). There were seven patients who received intraoperative RT (median dose 12.5 Gy, range 10–12.5 Gy) in addition to neoadjuvant RT.
Patient demographics are summarized on Table 1. There were no differences with regards to age, sex, ASA class, or BMI for patients in both groups. There were no differences between the two groups with regards to the tumor location, treated T or N stage, or preoperative CEA level (Table 2). Tumor size post resection, and neoadjuvant therapy did differ between groups (Table 2).
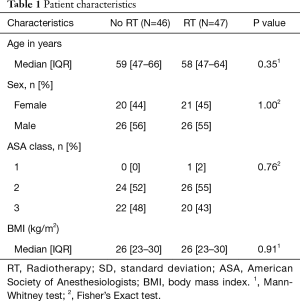
Full table
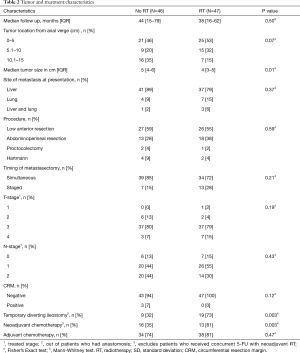
Full table
All patients underwent standard total mesorectal excision and complete resection of the metastatic lesion(s). There was no difference between the two groups with regards to the procedure performed or surgical margins status. More patients in the RT group had a temporary diverting stoma constructed (73% vs. 32%, P=0.003) (Table 2). Metastasectomy was predominantly performed simultaneously (72% for RT vs. 85% for no RT, P=0.21) (Table 2).
Oncologic outcomes
The long-term oncologic outcomes are summarized in Figures 1-3. The median follow-up for all patients was 43 months (IQR 16–67 months). The majority of recurrences occurred within 2 years of surgery (76%). Two patients (3.4%) developed a first recurrence 5 years following surgery. Figure 4 details the recurrence patterns and outcomes. No recurrence was evident at last follow-up or at death in 35% of patients in no RT group and 40% in RT group, P=0.67.

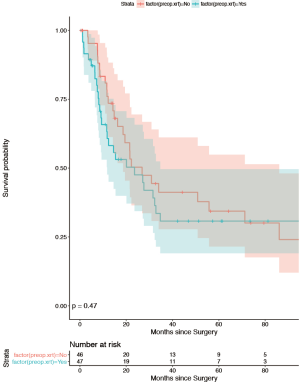
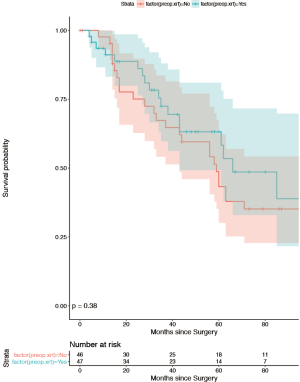
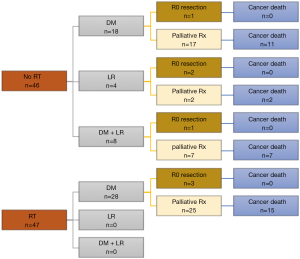
There were 12 patients (26%) who developed LR in the no RT group, while no LR (0%) was observed in the RT group (P<0.001, Figure 1). Four patients (8.5%) developed isolated LR, of which 2 had resection of LR and are disease-free. The other two patients died of their LR. Eight patients (17%) presented with concurrent LR and DM, of which seven died of their distant disease. The median time to LR was 13.5 months (range, 3–26 months). All patients who experienced LR had received systemic chemotherapy, before (n=1), after (n=8), or before and after (n=3) surgical resection. One patient with LR had a positive CRM. There were 10 LR in patients who had undergone low anterior resection and 2 in those with an APR. There were 26 patients (57%) in the no RT group who developed distant metastasis compared with 28 patients (60%) in the RT group, p=0.85. The median time to distant metastasis was 11.5 months (range, 1–85 months). Repeated metastasectomy was performed for seven patients (12%, 3 in the RT group and 4 in the no RT group). All patients who had surgery for recurrence are disease-free at last follow-up. OS, distant recurrence, and LR were stratified by year of surgery (1992–1999, 2000–2009, 2010–2014), and no difference was found in oncological outcomes. Moreover, even after stratifying for year of LR the difference between the RT and no RT groups remained significant, P<0.0001. The only difference found with stratification by years included the increasing use of neoadjuvant radiation [1992–1999 (no RT 64% vs. 36%), 2000–2004 (no RT 68% vs. 32%), 2005–2009 (no RT 44% vs. 56%], 2010–2014 (no RT 33% vs. 67%).
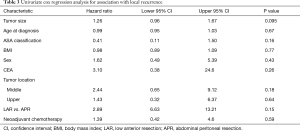
The omission of neoadjuvant RT was the only variable found to be associated with subsequent LR (P<0.0002). Univariate cox regression analysis found no association between age, sex, ASA class, BMI, Tumor size, nodal status, tumor location, procedure performed, or neoadjuvant chemotherapy, and subsequent LR (Table 3). The 5-year OS (Figure 3) rates were: 43.3% (95% CI: 30.1, 62.3%) (no RT) vs. 58.3% (95% CI: 43.4%, 78.2%) (RT). The 5-year DSS rates were 49.6% (95% CI: 35.5%, 69.4%) (no RT) vs. 60.5% (95% CI: 45.0%, 81.33%) (RT). Figures 1 and 2 illustrate LR and distant free recurrence curves.
Full table
Discussion
Neoadjuvant RT for non-metastatic locally-advanced rectal cancer is already the standard of care as it is associated with a lower risk of LR and improved functional results compared to adjuvant RT (14). Therefore, by limiting the analysis to patients who received neoadjuvant RT (excluding patients who received postoperative RT) we aimed to reflect current clinical practice and provide unique information in stage IV disease. The findings of this study demonstrated a reduction in LR rate for patients with stage 4 locally-advanced rectal cancer who underwent curative surgery and neoadjuvant radiotherapy. Neoadjuvant radiotherapy as expected appears to have no impact on the rate of distant metastasis or survival.
Despite the series small sample size, retrospective nature, prolonged time range, and potential for selection bias, the stringent inclusion criteria and long-term follow-up provide valuable information to fill knowledge gaps in potential future treatment algorithms for resectable metastatic rectal cancer. The study is limited due to its single institutional approach and potentially non generalizable conclusions because of the nature and complexity of stage IV disease. Moreover, it is possible that those patients who received neoadjuvant RT or chemotherapy were thought to represent a higher risk with potential clinical differences in height of tumor location and operation performed. Despite these limitations, we believe that this is the first study to clearly document and confirm the advantage of neoadjuvant RT for locally-advanced rectal cancer in the setting of resectable metastatic disease. Unfortunately, it remains unclear if a specific subgroup of patients would benefit more from neoadjuvant RT. Table 3 details the correlation between several potential perioperative risk variables for LR. However, this series was not designed to answer these questions definitively. This inability to select high risk patients suggest further study is needed.
Previous work may have failed to show benefit due to the heterogeneity of published studies. Most series include patients who had non-surgical management of synchronous metastasis and differences in timing of radiotherapy (10,15-20). The most relevant comparisons to our work involve two studies, which investigated the value of RT in patients undergoing curative surgery. Butte et al. (4) reported the outcomes of 185 patients with rectal cancer and early onset liver metastasis who underwent curative resection with the primary aim of reporting the patterns of recurrence. The LR rate in this series was 9% for patient who received perioperative RT and 8% for those who did not with the difference being insignificant. Similarly, Chen et al. (21) did not find an added benefit of adjuvant RT following curative surgery for rectal cancer with synchronous liver or lung metastasis. Ultimately, the potential benefit of radiotherapy is difficult to determine decisively from these series given the variation in timing of radiotherapy administration. Our study eliminates this limitation by focusing on locally-advanced tumors and patients who received neoadjuvant radiotherapy only. This may provide an explanation for our contrary findings to these two similar published studies (4,21). Moreover, the focus of our study on locally-advanced tumors increases the relevance of LR and the potential impact of radiotherapy when combined with proper surgery rather than a broader based stage IV cancer cohort. It is noteworthy to point out that a third series by Huh et al. (20) did find in their subgroup analysis that LR rate following resection of stage IV rectal cancer was statistically lower in patients who received neoadjuvant RT compared with those who received adjuvant RT.
Chemotherapy combined with surgery has been shown to improve the disease-free survival for patients with colorectal liver metastasis; the optimal timing of chemotherapy remains uncertain (22,23). New chemotherapy trials are underway which directly test the need for radiation at all (2). Likewise, stage IV disease has transitioned over time to include neoadjuvant chemotherapy as common practice in contrast to the standard postoperative therapy found before 2005. This finding is certainly consistent with our series. All patients who experienced LR had received perioperative systemic chemotherapy. Despite differences in neoadjuvant chemotherapy specifically, it was not associated with either an improvement in DSS or LR. However, it may be argued that this radiated cohort with a higher percentage of neoadjuvantly treated patients represents a higher risk cohort. If true, this may actually add credibility to our findings that despite higher risk these patients ultimately have similar distant recurrence rates with significantly improve LR. Ultimately, distant recurrence remains the factor most critical to long term outcomes for patients. Only seven out of 58 patients with recurrence of any kind (12%) were found to have resectable metastasis and underwent repeat curative metastasectomy and are alive at last follow-up (Figure 4).
Additionally, this series adds to our collective understanding of the nature of re-resection of LR. With an overall LR rate of 12.9% it is important to acknowledge that isolated LR was discovered in only 4.3%. These isolated LR were deemed resectable in 2 out of 4 patients. All of the patients who underwent resection of recurrence are disease-free, which affirms the importance of re-operative surgery in these patients when feasible (24). Unfortunately, LR as an isolated event was the cause of death in 2 out of those 4 patients or 2.2% of the total cohort. Of the eight patients with local and distant recurrence 7 eventually died of their distant disease. It has been successfully argued that it is distant disease, which remains the limitation on survival and that the impact of additional radiotherapy may only aid the minority of patients with isolated LR. Future efforts will be needed to understand proper selection criteria to determine who will benefit the most from this therapy. It is equally true that although there were no statistical differences in tumor height or operation performed there were clinically more APR and lower tumors in the radiated cohort. Radiation was a benefit to patients despite this finding of potential higher risk. Moreover, 10 of the LR occurred in Low anterior resection patients, which would potentially be considered as lower risk. This is demonstrated by a large HR 2.89 (Table 3) which was non-significant. This confirms the need for further risk stratification in order to avoid the potential negative impact radiotherapy may have on patients with low risk of LR.
The future implications on practice and research include identifying unique risk factors, which place patients with metastatic rectal cancer at highest risk for LR. Only then can we potentially modify those risks through combined chemoradiation therapy. Moreover, this data may aid providers by adding to the evidence, which supports the known benefits of neoadjuvant therapy for patients with advanced disease and allow for the translation of knowledge to metastatic disease.
Conclusions
In conclusion, as outcomes for stage IV rectal cancer continue to improve with modern therapy, the risk of LR needs to be included in the planning of optimal treatment strategy. We recommend consideration of neoadjuvant radiotherapy in advanced local rectal cancer with resectable stage IV disease until modifiable risk factors can be identified.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Mayo Clinic Institutional Review Board of 14-000439-01. All patients provided authorization for research.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [Crossref] [PubMed]
- Bossé D, Mercer J, Raissouni S, et al. PROSPECT Eligibility and Clinical Outcomes: Results From the Pan-Canadian Rectal Cancer Consortium. Clin Colorectal Cancer 2016;15:243-9. [Crossref] [PubMed]
- Weiser MR, Fichera A, Schrag D, et al. Progress in the PROSPECT trial: precision treatment for rectal cancer? Bull Am Coll Surg 2015;100:51-2. [PubMed]
- Butte JM, Gonen M, Ding P, et al. Patterns of failure in patients with early onset (synchronous) resectable liver metastases from rectal cancer. Cancer 2012;118:5414-23. [Crossref] [PubMed]
- Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6; discussion 6-7. [Crossref] [PubMed]
- Hur H, Ko YT, Min BS, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009;197:728-36. [Crossref] [PubMed]
- Lee WS, Yun SH, Chun HK, et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol 2008;42:945-9. [Crossref] [PubMed]
- Cho JH, Hamaji M, Allen MS, et al. The prognosis of pulmonary metastasectomy depends on the location of the primary colorectal cancer. Ann Thorac Surg 2014;98:1231-7. [Crossref] [PubMed]
- Rahbari NN, Elbers H, Askoxylakis V, et al. Neoadjuvant radiotherapy for rectal cancer: meta-analysis of randomized controlled trials. Ann Surg Oncol 2013;20:4169-82. [Crossref] [PubMed]
- Kim SH, Kim JH, Jung SH. Comparison of oncologic outcomes of metastatic rectal cancer patients with or without neoadjuvant chemoradiotherapy. Int J Colorectal Dis 2015;30:1193-9. [Crossref] [PubMed]
- New NCCN Guidelines Include Evidence Blocks to Illustrate Value in Breast, Colon, Kidney, and Rectal Cancers. J Natl Compr Canc Netw 2016;14:xxxiv-xxxv. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Andersen PK, Geskus RB, de Witte T, et al. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861-70. [Crossref] [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Kim JW, Kim YB, Kim NK, et al. The role of adjuvant pelvic radiotherapy in rectal cancer with synchronous liver metastasis: a retrospective study. Radiat Oncol 2010;5:75. [Crossref] [PubMed]
- Manyam BV, Mallick IH, Abdel-Wahab MM, et al. The Impact of Preoperative Radiation Therapy on Locoregional Recurrence in Patients with Stage IV Rectal Cancer Treated with Definitive Surgical Resection and Contemporary Chemotherapy. J Gastrointest Surg 2015;19:1676-83. [Crossref] [PubMed]
- Lin JK, Lee LK, Chen WS, et al. Concurrent chemoradiotherapy followed by metastasectomy converts to survival benefit in stage IV rectum cancer. J Gastrointest Surg 2012;16:1888-96. [Crossref] [PubMed]
- Lee JH, Jo IY, Yoon SC, et al. The role of postoperative pelvic radiation in stage IV rectal cancer after resection of primary tumor. Radiat Oncol J 2012;30:205-12. [Crossref] [PubMed]
- Chang CY, Kim HC, Park YS, et al. The effect of postoperative pelvic irradiation after complete resection of metastatic rectal cancer. J Surg Oncol 2012;105:244-8. [Crossref] [PubMed]
- Huh JW, Kim HC, Park HC, et al. Is Chemoradiotherapy Beneficial for Stage IV Rectal Cancer? Oncology 2015;89:14-22. [Crossref] [PubMed]
- Chen J, Wang DR, Yu HF, et al. Defunctioning stoma in low anterior resection for rectal cancer: a meta- analysis of five recent studies. Hepatogastroenterology 2012;59:1828-31. [PubMed]
- Araujo R, Gonen M, Allen P, et al. Comparison between perioperative and postoperative chemotherapy after potentially curative hepatic resection for metastatic colorectal cancer. Ann Surg Oncol 2013;20:4312-21. [Crossref] [PubMed]
- Ciliberto D, Prati U, Roveda L, et al. Role of systemic chemotherapy in the management of resected or resectable colorectal liver metastases: a systematic review and meta-analysis of randomized controlled trials. Oncol Rep 2012;27:1849-56. [PubMed]
- Assumpcao L, Choti MA, Gleisner AL, et al. Patterns of recurrence following liver resection for colorectal metastases: effect of primary rectal tumor site. Arch Surg 2008;143:743-9; discussion 9-50. [Crossref] [PubMed]


