|
Original Article
Radiation therapy in the postoperative management of esophageal cancer
Salma K Jabbour1, Charles R Thomas Jr2
1Department of Radiation Oncology, Cancer Institute of New Jersey, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, New Bruswick, NJ, USA; 2Department of Radiation Medicine, Oregon Health & Science University, Portland, OR, USA
Corresponding author: Charles R Thomas Jr, MD, Professor and Chair. Department of Radiation Medicine, Oregon Health and Science University, Knight Cancer Institute, Mail Code KPV4, 3181 SW Sam Jackson Park Road, Portland, OR 97239-3098, USA. Fax: 503-346-0237; Tel: 503-494-8756. Email: thomasch@ohsu.edu.
|
|
Abstract
The optimal management of esophageal cancer is complicated since institutional preferences vary, patient characteristics often guide management, and there are data to support multiple approaches for locally advanced esophageal cancer. Although surgery is an important component of therapy, alone it results in unacceptably high rates of local relapse and poor long-term survival rates. Well-studied adjuvant approaches include upfront chemoradiation therapy with or without surgery, perioperative chemotherapy, adjuvant radiation or adjuvant chemoradiation. This review article seeks to examine thoroughly the role of postoperative therapeutic options for the management of esophageal cancer, and in so doing, also overviews prospective trials in the neoadjuvant, definitive and perioperative settings. Studies evaluating radiation field design are also discussed.
Key words
esophageal cancer; radiation therapy; postoperative; chemoradiation therapy
J Gastrointest Oncol 2010; 1: 102-111. DOI: 10.3978/j.issn.2078-6891.2010.013
|
|
Introduction
Esophageal cancer is a rare disease with a poor prognosis,
accounting for approximately 1% of all malignancies, with
an estimated 16,640 cases in 2010 and 14,500 deaths ( 1).
In the United States, the incidence of adenocarcinoma
has risen, while squamous cell carcinoma has declined. It
is now recognized in the AJCC staging system that these
two histologies can carry different clinical outcomes ( 2).
Institutional preferences and patient characteristics will often
guide the management, as there are data to support multiple
approaches for locally advanced esophageal cancer including
upfront chemoradiation therapy (CRT) with or without
surgery, perioperative chemotherapy, adjuvant radiation or
chemoradiation. Surgery generally remains a mainstay in
management of localized esophageal cancer, but as a single modality results in unacceptably high rates of local relapse
and poor long-term survival rates, leading to the integration
of radiation therapy and chemotherapy as neoadjuvant or
adjuvant modalities. The results of many studies have led
to mixed results; therefore, there is no consensus about the
optimal management of these patients. There is a growing recognition that even in well clinically
stage ultrasound T2 N0 esophageal cancer, between 20-25%
may be upstaged to have pathologic T3 and/or node positive
disease. Hence, these patients would often be referred for
postoperative therapy. This review, while addressing the
different sequencing of multimodality therapy, aims to focus
mostly on how best to manage patients in the postoperative
setting.
|
|
Definitive chemoradiotherapy
Along the lines of definitive management of esophageal
cancer, it is important to discuss the RTOG 8501 trial
which was instrumental in defining the superiority of
chemoradiation over radiation therapy ( 3). The trial
randomized patients to 64 Gy alone (n=60) to 50 Gy with
concurrent cisplatin and 5-FU (n=61) for a total of 4 courses
of chemotherapy. Overall survival at 2 years increased from
10% with radiation alone to 38% in the combined therapy
group (p=0.001). Distant and local recurrences were also
reduced in the chemoradiation group. An update of this study showed that the 5-year survival rate with CRT was 27%
compared to 0% with radiation alone ( 4). Approximately
85% of these patients had squamous histology. Of note, the
2010 NCCN guidelines recommend that T1 node positive or
T2-T4 Nx esophageal cancer cases be treated with definitive
chemoradiation or preoperative chemoradiation (50-50.4 Gy)
followed by either esophagectomy (preferred) or observation
for those achieving a complete clinical response, or for
those with persistent local disease, either esophagectomy
(preferred) or palliative treatment. It is recommended
adenocarcinoma of the distal esophagus or GEJ be treated
with preoperative chemotherapy followed by esophagectomy.
|
|
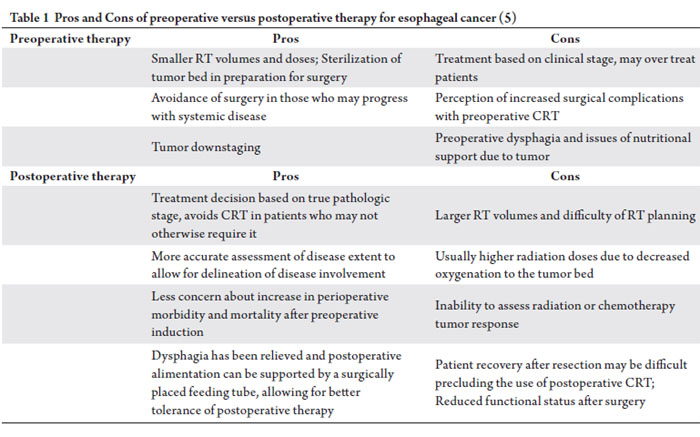
Preoperative versus postoperative therapy
From a radiotherapeutic standpoint, preoperative irradiation
is advantageous compared to postoperative irradiation,
because of an intact vascular supply allowing for improved
oxygenation, generally smaller radiation portals and lesser
radiation doses, sterilization of the operative bed, avoidance
of surgery in patients with aggressive disease, and tumor
downstaging. The advantage of postoperative therapy is the
knowledge of the pathological stage to appropriately select
patients for therapy. The pros and cons of preoperative versus
postoperative therapy are further discussed in Table 1.
With preoperative therapy, optimal tumor downstaging can result in complete pathological response of the tumor,
portending improved survival outcomes for esophageal
carcinoma. Pathological complete response (pCR) has often
been used as a surrogate for efficacy of therapy and a measure
by which various neoadjuvant therapies in esophageal
cancer can be compared. Rohatgi et al retrospectively
analyzed 235 patients who underwent preoperative CRT for
adenocarcinoma (82%) or squamous cell (18%) carcinoma of
the esophagus and found that patients who experienced pCR
had longer overall and disease free survival rates, fewer distant
metastases, and less disease recurrences ( 6). At 37-month
follow-up, patients with pCR had a 74% overall survival,
compared to 65% for those with < 50% residual disease after
CRT, and 40% for those with > 50% residual disease after
CRT. In addition, pCR may be more predictive of survival for
patients with adenocarcinoma than squamous cell carcinoma
in those receiving preoperative CRT ( 7).
|
|
Preoperative chemotherapy
Investigators have evaluated multiple neoadjuvant regimens
consisting of preoperative chemotherapy or perioperative
chemotherapy. Despite the available studies, biases may still
remain about the benefit of perioperative chemotherapy
versus CRT. RTOG 8911 compared surgery alone with
chemotherapy followed by surgery, revealing no overall survival difference between the two arms. Patients who
underwent less than an R0 resection had an ominous
prognosis (5-year overall survival for R0 resection 32%, and
R1 resection 5%) ( 8). Cunningham et al evaluated surgery
alone compared to a regimen consisting of 3 cycles of both
preoperative and postoperative epirubicin, cisplatin, and
5-fluorouracil (ECF) for resectable gastroesophageal cancer
and showed significant downstaging, but pathological
complete response rates were zero. With the addition of
chemotherapy, 5-year survival was improved from 23%
to 36% with chemotherapy and progression free survival
was also significantly improved ( 9). The Medical Research
Council also demonstrated a significant 2-year overall
survival benefit from 34% to 43% with the addition of 2
cycles of preoperative cisplatin and 5-FU (p=0.004) ( 10).
A meta-analysis by Urschel et al evaluated 11 randomized
clinical trials including nearly 2,000 patients treated with
neoadjuvant chemotherapy compared to surgery alone ( 11).
Although higher rates of complete resection (R0) were seen
with preoperative chemotherapy, no survival benefit was
seen for combined chemotherapy and surgery. Preoperative
chemotherapy is considered a standard option for resectable
adenocarcinoma of the GEJ but remains controversial for the
preoperative management of intrathoracic esophageal cancer.
|
|
Preoperative chemoradiotherapy versus surgery alone
Surgery is considered important in the management of
esophageal cancers. The CALGB 9781 study randomized
esophageal cancer patients (77% adenocarcinoma, 24%
squamous cell carcinoma) to preoperative chemoradiation
(cisplatin, 5-FU, and RT to 50.4 Gy) followed by surgery
versus surgery alone ( 12). Despite poor accrual (56 out of a
planned 475 patients), a significant survival advantage was
seen in the trimodality group with 5-year survival of 39%
versus 16% with surgery alone and median survival of 4.5
years compared to 1.8 years with surgery alone (p=0.002).
The addition of chemoradiation in this setting afforded a
convincing survival benefit and provided justification for the
existing de-facto standard of care in patients with clinical stage
II-III disease. In an EORTC study reported by Bosset, 282 patients with
squamous cell carcinoma were randomized to preoperative
cisplatin with radiation therapy (split course 37 Gy using 3.7
Gy per fraction) followed by surgery versus surgery alone
( 13). Results showed significant improvements in favor of
preoperative therapy for disease-free survival, local control,
cancer-related deaths, and curative resection rates; however,
there was no difference in overall survival (18.6 months
for both groups). Significantly more postoperative deaths were seen in the group treated with preoperative CRT (12%
versus 4% with surgery alone), mainly because of the higher
number of patients with respiratory insufficiency, mediastinal
infection or sepsis. The authors discussed that the increased
number of postoperative deaths in the CRT could have been
due to the “deleterious effects of high dose of radiation per
fraction or of CRT on lung tissue.” They recommended future
studies incorporate 2-Gy range fraction sizes, continuous
radiation to overcome repopulation seen with split course
therapy, and 5-FU chemotherapy. This trial therefore showed
that preoperative CRT could prolong disease-free survival
and local control but not overall survival although was likely
limited by the radiation scheme. An Australian study by Burmeister et al evaluated 257
patients with both adenocarcinoma (63%) and squamous
cell carcinoma (27%) of the esophagus ( 14). Patients
were randomized to preoperative cisplatin and 5-FU with
concurrent radiation therapy (35 Gy in 15 fractions) or
immediate surgical resection. The CRT and surgery groups
had significantly more complete resections with clear margins
and fewer positive lymph nodes than the surgery alone group
did. However, neither progression-free survival (16 months
with CRT and surgery versus 12 months with surgery alone,
HR=0.82, p=0.32) nor overall survival (22 months with CRT
and surgery versus 19 months with surgery alone, HR= 0.89,
p=0.57) differed between the groups. On subset analysis,
patient with squamous cell tumors had a better progressionfree
survival with CRT (HR 0.47, p=0.014) than those with
non-squamous tumors (HR=1.02, p=0.92). Weaknesses
of this trial included administration of only one cycle of
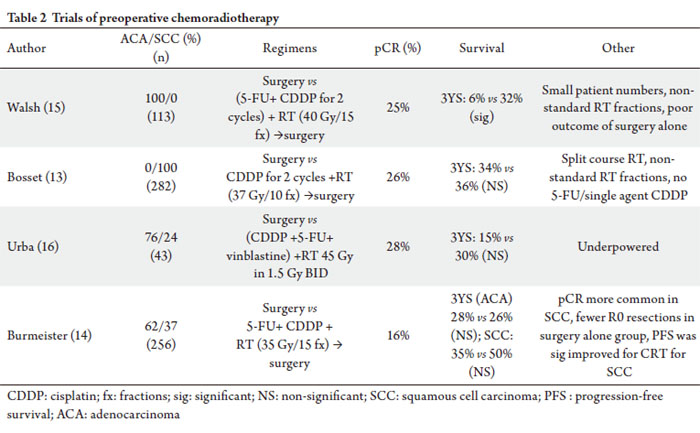
chemotherapy and relatively low radiation doses. Multiple trials have evaluated preoperative chemoradiation
therapy with some improvement in survival outcomes and
notable pathological complete response rates as detailed in
Table 2.
|
|
Preoperative chemoradiotherapy versus definitive chemoradiotherapy
Some authorities believe that the role of surgery for squamous
cell carcinomas remains controversial based on two studies,
one from France and another from Germany. The Federation
Francophone de Cancerologie Digestive Study 9102 enrolled
444 patients with resectable squamous cell carcinoma (89%)
or adenocarcinoma (11%), to receive one of two radiation
schemes with 2 courses of concurrent cisplatin and 5-FU:
1) protracted radiotherapy (46 Gy over 4.5 weeks) (64%
of participants) or 2) split course radiotherapy with two
1-week courses of 15 Gy with a 2 week break (36%) ( 17).
259 patients who responded to therapy were randomly
assigned to surgery or additional chemoradiation. For the non-responders, they continued on a course of CRT with
an additional 20 Gy for the protracted course and 15 Gy for
the split course CRT. No significant differences were seen in
median survival and (17.7 months in those who underwent
surgery compared to 19.3 months in the definitive CRT arm)
2-year survival (34% in surgery cohort vs 40% in the CRT
arm, p=0.44). Nevertheless, the 2-year local control rate was
higher with surgery (66%) compared to CRT (57%). The
3-month mortality rate was 9% in the surgery group and
1% in the CRT group. The results of this trial imply that for
patients who respond to CRT, surgery may improve local
control but not survival. In a similar study design by Stahl et al, 172 patients with
locally advanced squamous cell carcinoma of the esophagus
were randomized to either induction chemotherapy (5-FU,
leucovorin, etoposide, and cisplatin for 3 cycles) followed
by CRT (40 Gy with cisplatin and etoposide) followed by
surgery or the same induction chemotherapy followed by
CRT (total dose of 60-65 Gy with or without brachytherapy)
without surgery ( 18). Overall survival at 2-years (40%
with surgery vs 35% with CRT) and median survivals (16
months vs 15 months) were equivalent. Freedom from
local progression was improved with surgery (64% vs 41%,
p=0.003). Surgery improved outcomes for non-responders
to CRT who had 3-year survival rates of 18% with surgery
compared to 9% with CRT alone. Treatment related mortality was also higher in the surgery arm (13% vs 3.5%, p=0.03).
The addition of surgery to CRT improved tumor control but
not survival for squamous cell carcinomas. Because many of the randomized clinical tr ials
investigating surgery versus preoperative therapy have been
underpowered, meta-analyses have been performed. Gebski
et al showed a 13% absolute survival benefit at 2 years with
the neoadjuvant CRT (hazard ratio 0.81, p=0.02) with
similar results for squamous cell carcinoma (hazard ratio
of 0.84, p=0.04) and adenocarcinoma (hazard ratio 0.75,
p=0.02). Neoadjuvant chemotherapy portended a 2-year
absolute survival benefit of 7% with only a significant effect
on all-cause mortality for adenocarcinoma of the esophagus
and not squamous cell carcinoma ( 19). Urschel et al also
demonstrated improved 3-year survival, higher rates of R0
resection and tumor downstaging, and reduced local-regional
recurrence with neoadjuvant CRT compared to surgery alone
( 20, 21). In sum, there does appear to be a survival benefit
with the addition of CRT to surgery. |
|
Adjuvant (postoperative) therapy
The goal of adjuvant radiation therapy for esophageal cancer
is to decrease the risk of locoregional recurrence and in so
doing, can contribute to a survival benefit. As noted earlier,
it is not uncommon for patients with clinically staged ultrasound T2 N0 diseased to be upstaged to pathologic T3
or node positive status following resection ( 22). Rationale
for postoperative radiotherapy includes advanced tumor stage
(T3 or T4), nodal positivity, positive margins, or subtotal
resection ( 23). |
|
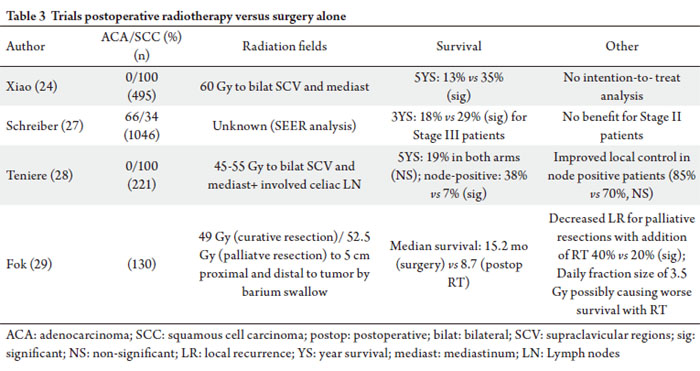
Postoperative radiation therapy versus surgery alone
Most of the series which will be discussed in the upcoming
sections are based on populations of squamous cell carcinoma
of the esophagus. There is a clear benefit in local control with
the addition of radiation and possibly a survival advantage.
However, many of these studies were conducted prior to the
advent of PET staging by which we now can identify 10-15%
of patients with occult metastatic disease which may change
their management and survival outcomes.
The largest of these series is by Xiao and included 495
patients with squamous cell carcinoma of the esophagus
who received postoperative radiation therapy (n=220) or
surgery alone (n=275) ( 24). Radiation portals encompassed
the bilateral supraclavicular areas and entire mediastinum to
a total of 60 Gy (40 Gy prescribed to midplane and 20 Gy
from horizontal portals, treated over 6 weeks). Survival was
improved non-significantly with the addition of RT from 32%
to 41% (p=0.45). Stage III patients had a distinct, significant
overall survival improvement with the addition of RT from
13% to 35% at 5 years (p=0.003). This trial has been criticized
for not employing an intention-to-treat analysis, since it
excluded 54 patients who did not complete the planned
course of treatment. The lack of informed patient consent
called into question the ethical standards of this trial ( 25). In a separate retrospective analysis by Xiao et al by extent
of lymph node status, 549 patients were classified into three
groups: Group 1: no lymph node involvement, Group 2: onetwo
positive lymph nodes, Group 3: three or more positive
lymph nodes. The 5-year survival rate of patients with positive
lymph nodes (Groups 2 and 3) was 18% with surgery alone
compared to 34% with the addition of RT (p=0.038) ( 26).
Also, for similar stage III patients, the number of lymph nodes
predicted survival outcomes with 5-year survival at 58% for
group 1, 31% for Group 2, and 14% for Group 3. Although
there was no survival benefit for lymph node negative
patients, those with one to two positive lymph nodes had an
improvement in 5-year overall survival with the addition of
RT from 24% to 45%. For patients with 3 or more positive
lymph nodes, 5-year survival outcomes were 21% with RT
versus no survivors with surgery alone. Not only is number
of metastatic lymph nodes prognostic, but the addition of RT
improved survival in patients with positive lymph nodes. An analysis of the Surveillance Epidemiology and End
Results (SEER) database evaluated the impact of adjuvant radiation in 1046 patients, who received surgery alone (65%)
or postoperative radiation (35%) ( 27). For Stage III patients
there was significant improvement in median (15 to 19
months), 3-year overall survival (18 to 29%) (p< 0.001), and
disease specific survival (18 to 24 months) (p< 0.001) which
was present for both adenocarcinoma and squamous cell
carcinomas. No improvement in survival was seen with Stage
II esophageal cancer (AJCC 6 th edition) with the addition of
postoperative RT. Multivariate analysis also confirmed that
the addition of adjuvant RT was associated with an improved
survival (HR 0.70, 95% CI 0.59-0.83, p<0.001). This analysis
is limited by the lack of information about chemotherapy,
radiation fields and doses, and margin status. Teniere et al evaluated patients with squamous cell
carcinoma of the middle to lower third of the esophagus
and randomized them to obser vation (n=102) or
postoperative RT (n=119) (45-55 Gy in 1.8 Gy per fraction
to the bilateral supraclavicular regions, mediastinum, and
involved celiac lymph nodes) ( 28). Patients were stratified
by nodal involvement extent. Five-year survival in node
negative patients was 38% versus 7% with involved nodes.
Postoperative RT did not confer a survival benefit (5-year
survival of 19% in both arms). Rates of local regional
recurrence were lower in patients receiving postoperative
radiation versus surgery alone (85% vs 70%) but not
statistically significant. Patients without nodal involvement
did have significant improvement in local regional recurrence
with the addition of radiation therapy (90% vs 65%). Fok et al included both squamous cell carcinoma and
adenocarcinoma histologies in their study and stratified
patients based on palliative (n=70) versus curative (n=60)
resection prior to randomization to postoperative RT versus
observation ( 29). Prescribed radiation doses of 49 Gy for
curative resection and 52.5 Gy for palliative resection in 3.5
Gy per fraction were used, delivered to a 5 cm margin both
proximal and distal to the initial tumor extent as delineated
by barium swallow. Although they demonstrated a decline
in local recurrence rates for those who underwent palliative
resection followed by adjuvant RT (20% postoperative RT,
46% no RT, p=0.04), there was no statistical difference in
local recurrence for those who had complete resection (15%
with RT versus 31% with surgery alone, p=0.06). The overall
median survival was significantly shorter for patients receiving
postoperative RT (8.7 months) versus control (15.2 months).
In patients with residual tumor in the mediastinum after
resection, two died of tracheobronchial obstruction compared
to nine in the control group. The authors concluded that the
shorter survival of patients who underwent postoperative
radiotherapy was the result of irradiation-related death
and the early appearance of metastatic disease, although
patients were less likely to have a recurrence obstructing the tracheobronchial tree. The major criticism of this trial has
been the large fraction sizes and total dose delivered which
may have contributed to the increased mortality rates and
resulted in substantially higher gastric pull-up complications
(37% with RT versus 6% with surgery alone) and six fatal
bleeding events in the RT group. Similarly, Zieren et al
evaluated 68 squamous cell carcinoma patients who were
randomized to either observation or postoperative RT,
finding no difference in overall or disease-free survivals, but
an increase in fibrotic esophageal strictures in the RT arm
( 30). In a meta-analysis of postoperative radiotherapy
trials, no significant difference in the risk of mortality
with postoperative radiotherapy and surgery at one year
compared with surgery alone was detected (RR, 1.23; 95%
CI, 0.95 to 1.59; p = 0.11) ( 31). The rate of local recurrence
with radiotherapy was lower in the tirals of Xiao and Fok
( 24, 29), but the two trials of Teniere and Zieren ( 28, 30)
noted this benefit was achieved at the expense of increased
morbidity. Given modern day techniques, improved treatment
planning with str ict dose volume histogram data ,
postoperative RT is expected to be safer with less toxicity
than previous studies. Based on the aforementioned studies,
improvements in local control can be expected and is
particularly important in the setting of nodal positivity or
R1/R2 resection.
|
|
Postoperative radiation therapy versus postoperative chemo-therapy
The Japanese Esophageal Oncology Group evaluated
postoperative radiotherapy (50 Gy to supraclavicular
regions and upper mediastinum in 2 Gy/day) versus 2
cycles of cisplatin and vindesine ( 32). Of the 258 patients
randomized, 73% had positive lymph nodes and 65-70%
of patients had T3 or T4 disease, but histology was not
delineated. Overall survival was no different (3-year
survival rates were 51% (RT) and 52% (chemotherapy)
and local recurrence rates were also equivalent. In contrast,
in a retrospective study by Chen et al of 366 patients with
squamous cell carcinoma of the mid-thoracic esophagus,
local recurrence rates were significantly lower with adjuvant
radiation therapy compared to chemotherapy or observation
(20%, 32%, 43%, respectively) ( 33). |
|
Postoperative chemoradiation versus surgery alone
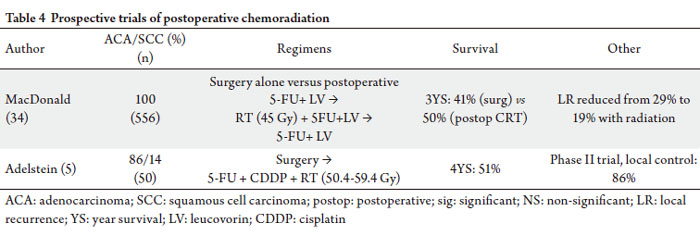
The INT-0116 trial published by MacDonald et al
prospectively randomized 556 patients with gastroesophageal
junc t ion (GEJ) (approx imately 20%) or gastric
adenocarcinoma patients, Stage IB-IV (AJCC 3 rd Edition)
who had undergone curative resection with negative
margins to receive no further therapy or to postoperative
chemoradiation (one cycle of 5-FU and leucovorin followed
by concurrent radiation to 45 Gy with the same agents,
followed by two additional cycles of 5-FU and leucovorin)
( 34). Patients were required to have sufficient caloric intake of 1500 Kcal per day. Because of the complicated nature of RT
field design for gastric carcinomas, RT quality assurance was
conducted prior to radiation delivery, and both minor and
major deviations were detected in 35% of cases and corrected.
Three-year overall survival improved with addition of
chemoradiation from 41% to 50% as well as median survival
from 27 months to 36 months with chemoradiation. (HR 1.35
for death with surgery alone group compared to adjuvant
CRT, 95% CI 1.09-1.66, p=0.005). Local recurrence rates
were also reduced from 29% with surgery alone to 19% with
the addition of CRT. This trial provides the rationale for
the use of postoperative CRT for GEJ adenocarcinomas. In
patients with GEJ adenocarcinomas, CRT is appropriate to
improve survival and local control. Of note, in the 6 th Edition of the AJCC manual, GEJ
carcinomas could be included in esophageal or gastric stage
groupings and could produce different stage groupings
depending on either the use of the esophageal or gastric
stage groupings. GEJ carcinoma also previously included
the locally advanced stages of T4 Nx or Tx N3 (Stage IV as
stated above) when grouped with gastric cancer ( 35). In the
AJCC 7 th Edition, the GEJ carcinomas are now staged with
esophageal, rather than gastric cancers, and include cancer
within the first 5 cm of the stomach that extends into the
GEJ or distal thoracic esophagus ( 2, 36). In addition, Stage
IV disease currently only refers to M1 staging and does not
include any locally advanced disease. A phase II trial of postoperative CRT for poor prognosis
esophagus and GEJ adenocarcinoma (86%) and squamous
cell carcinomas (14%) investigated postoperative 5-FU,
cisplatin and RT to 50.4-59.4 Gy in 50 patients with node
positive or T3/T4 tumors ( 5). 4-year freedom from
recurrence was 50%, distant metastatic control 56%, and
locoregional control 86%, with a median survival of 53
months, comparing favorably with a historical median
survival of 28 months in prior trials ( 37). Bedard et al retrospectively evaluated 28 node positive patients treated with surgery alone compared to 38 patients
treated with surgery and postoperative CRT. There were
more local recurrences with surgery alone (35% versus 13%
with CRT, p=0.09) ( 38). Overall survival was significantly
improved with postoperative CRT, and median survival
was 47.5 months with CRT versus 14.1 months with
surgery alone. Similarly, Rice et al, on retrospective analysis,
demonstrated a 28-month with CRT versus 14-month
median survival with surgery alone ( 37, 39). In modern day practice, it would reasonable to add
chemotherapy to postoperative radiation therapy as per
NCCN guidelines, to maximize the benefit of radiosensization
with systemic therapy, especially if the patient could tolerate
such a course. The available data do suggest that postoperative
RT alone also would be appropriate. For adenocarcinomas of
the GEJ, the MacDonald protocol is reasonable.
|
|
Postoperative chemoradiation versus postoperative
radiation therapy alone
A non-randomized prospective study from Taiwan evaluated
postoperative patients with T3-4 and N0-1 esophageal
carcinoma who were assigned to either CRT with weekly
cisplatin followed by adjuvant chemotherapy consisting of
cisplatin and 5-FU for four cycles (n=30) or postoperative
RT alone (n=30) ( 39). RT was delivered to 55-60 Gy in both
arms. A significantly better overall survival was seen with CRT
(31 months vs 21 months) and 3-year survival was improved
to 70% with CRT versus 34% with RT alone (p=0.003). |
|
Radiation therapy field design
Patients undergo a simulation with a contrast-enhanced
computed tomographic (CT) scan, in the treatment position
along with an immobilization device, usually in a supine
position. Many investigators are utilizing four-dimensional
CT scans ( 40). Appreciation of how the post-resection esophageal conduit moves with respiration, will aid the
radiation oncologist in developing portals that cover sites at
highest risk for loco-regional recurrence. In pathological analysis of patients with esophageal
and GEJ carcinoma, Gao et al prospectively collected and
evaluated 34 squamous cell carcinomas and 32 carcinomas
of the GEJ to assess microscopic spread both proximally and
distally in the specimens ( 41). For squamous cell carcinomas,
mean microscopic tumor extension beyond the gross tumor
was found to be 10.5 + 13.5 mm proximally (<30 mm in
94%) and 10.6 + 8.1 mm distally (<30 mm in 97%). In GEJ
adenocarcinomas, the spread was 10.3 + 7.2 mm proximally
(<30 mm in all cases) and 18.3 + 16.3 mm distally (<30 mm
in 84%). Lymph node metastases were observed in 35%
of patients with middle and lower esophageal squamous
cell carcinomas and 47% of patients with GEJ carcinomas.
The recommended Clinical Target Volume (CTV) margin
was <30 mm in about 94% of esophageal cancers (pleural),
except for distal microscopic spread in GEJ adenocarcinomas
(pleural), in which 50 mm was needed to cover 94% of cases. In a comparison of efficacy of regional and extensive clinical target volumes in postoperative radiotherapy for
esophageal squamous cell carcinoma, 102 patients with
T3/T4 or N1 disease treated with >50Gy were reviewed
( 42). In extensive portal irradiation (n=43) cohort, the
CTV encompassed the bilateral supraclavicular regions, all
mediastinal lymph nodes, the anastomotic sites, and the left
gastric and pericardial lymphatics. In the regional irradiation
group (n=59), the CTV was confined to the tumor bed and
the lymph nodes in the immediate region of the primary
lesion. The 1-, 3-, and 5-year survival rates between the two
groups were nearly identical. It is appropriate to use a regional
portal which affords similar survival outcomes to an extended
field and less acute and long-term toxicity. At the University of Erlangen, Meier et al, analyzed
patterns of regional spread using pathology reports of 326
patients with adenocarcinoma of the GEJ who had undergone
primary resection with >15 lymph nodes examined ( 43) .
Tumors were classified into Type I (distal esophagus), Type
II (cardia), and Type III (subcardial) based on pathology
and endoscopy reports. Marked esophageal invasion of GEJ
Type II and III significantly correlated with paraesophageal nodal disease, and T3-T4 Type II/III had a significant rate
of splenic hilum/artery nodes. Therefore, middle and lower
paraesophageal nodes should be treated in T2-T4 Type I and
II with > 15 mm of involvement above the Z line, and T3-T4
Type II. In addition, a study from Japan, in which 102 of cases
were examined (85% squamous cell carcinoma), showed that
the rates of lymph node metastases for the upper, middle,
lower and abdominal esophagus were 37.5%, 32.5%, 46% and
70%, respectively ( 44). It is helpful to know which lymph nodal stations are
involved with metastatic disease in order to develop
rationale field designs ( 41). Positive nodes may be seen
in approximately one-third of resected middle and lower
esophageal SCCA cases, with the subcardial, paraesophageal,
and left gastric nodal stations being the most common
sites ( 41). Distal adenocarcinoma lesions may harbor node
positive disease almost half of the time with the left gastric
and para-cardiac nodal stations being the most common
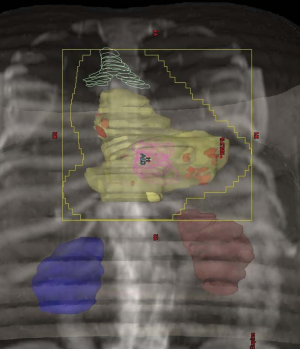
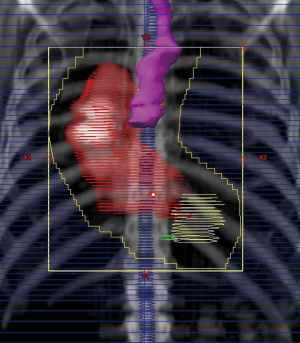
(Figure 1 and 2). In the postoperative setting, it seems reasonable to treat
a regional field encompassing the preoperative intrathoracic
esophageal tumor volume with a 3 cm cephalad and caudal
margin for the clinical target volume (CTV), and 3-5 cm
cephalad and caudal margins for GEJ carcinomas. Regional
lymph nodes will also be treated as well as anastomotic sites.
If daily image guidance techniques, such cone-beam CT
scans are utilized, it may be possible to reduce the planning
target volume (PTV). Postoperative doses of 45-50.4 Gy
for R0 complete surgical resection with negative margins
are appropriate to reduce long-term complications such as
stricture. Higher doses of 54-60 Gy would be recommended
for patients with R1 resections.
|
|
Conclusions
Adjuvant chemoradiation is a suitable option for the
management of the resected, locally advanced esophageal
cancer patient, especially for T3/T4 disease, nodal positivity,
and R1 or R2 resection. Doses of 45 to 50.4 Gy can be used
for R0 to R1 resections, but for gross residual disease, a boost
of 5-9 Gy may be considered. For tumors of the intrathoracic
esophagus, concurrent cisplatin and 5-FU can be used,
and for GEJ carcinomas, the INT-0116 protocol can be
recommended. The available data suggests an improvement
in local control and a possible survival improvement with the
use of postoperative radiation therapy.
|
|
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin
2010;60:277-300.[LinkOut]
- Greene FL, Trotti A, Fritz AG, Compton C, Byrd D, Edge S, editors. AJCC
Cancer Staging Manual. 7th ed. New York: Springer-Verlag; 2009.
- Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius
V, et al. Combined chemotherapy and radiotherapy compared with
radiotherapy alone in patients with cancer of the esophagus. N Engl J Med
1992;326:1593-8.[LinkOut]
- al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle JS, Vaitkevicius
VK, et al. Progress report of combined chemoradiotherapy versus
radiotherapy alone in patients with esophageal cancer: an intergroup study.
J Clin Oncol 1997;15:277-84.[LinkOut]
- Adelstein DJ, Rice TW, Rybicki LA, Saxton JP, Videtic GM, Murthy SC,
et al. Mature results from a phase II trial of postoperative concurrent
chemoradiotherapy for poor prognosis cancer of the esophagus and
gastroesophageal junction. J Thorac Oncol 2009;4:1264-9.[LinkOut]
- Rohatgi P, Swisher SG, Correa AM, Wu TT, Liao Z, Komaki R, et al.
Characterization of pathologic complete response after preoperative
chemoradiotherapy in carcinoma of the esophagus and outcome after
pathologic complete response. Cancer 2005;104:2365-72.[LinkOut]
- Rohatgi PR, Swisher SG, Correa AM, Wu TT, Liao Z, Komaki R, et
al. Histologic subtypes as determinants of outcome in esophageal
carcinoma patients with pathologic complete response after preoperative
chemoradiotherapy. Cancer 2006;106:552-8.[LinkOut]
- Kelsen DP, Winter KA, Gunderson LL, Mortimer J, Estes NC, Haller
DG, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113):
a random assignment trial comparison of chemotherapy followed by
surgery compared with surgery alone for esophageal cancer. J Clin Oncol
2007;25:3719-25.[LinkOut]
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ,
Nicolson M, et al. Perioperative chemotherapy versus surgery alone for
resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20.[LinkOut]
- Medical Research Council Oesophageal Cancer Working Group. Surgical
resection with or without preoperative chemotherapy in oesophageal
cancer: a randomised controlled trial. Lancet 2002;359:1727-33.[LinkOut]
- Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled
trials that compared neoadjuvant chemotherapy and surgery to surgery
alone for resectable esophageal cancer. Am J Surg 2002;183:274-9.[LinkOut]
- Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R,
et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil,
radiotherapy, and surgery compared with surgery alone for esophageal
cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92.[LinkOut]
- Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, et al.
Chemoradiotherapy followed by surgery compared with surgery alone in
squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7.[LinkOut]
- Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt
P, et al. Surgery alone versus chemoradiotherapy followed by surgery for
resectable cancer of the oesophagus: a randomised controlled phase III
trial. Lancet Oncol 2005;6:659-68.[LinkOut]
- Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy
TP. A comparison of multimodal therapy and surgery for esophageal
adenocarcinoma. N Engl J Med 1996;335:462-7.[LinkOut]
- Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery
alone in patients with locoregional esophageal carcinoma. J Clin Oncol
2001;19:305-13.[LinkOut]
- Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, et al.
Chemoradiation followed by surgery compared with chemoradiation
alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol
2007;25:1160-8.[LinkOut]
- Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al.
Chemoradiation with and without surgery in patients with locally advanced
squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7.[LinkOut]
- Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival
benefits from neoadjuvant chemoradiotherapy or chemotherapy in
oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34.[LinkOut]
- Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that
compared neoadjuvant chemoradiation and surgery to surgery alone for
resectable esophageal cancer. Am J Surg 2003;185:538-43.[LinkOut]
- Fiorica F, Di Bona D, Schepis F, Licata A, Shahied L, Venturi A, et al.
Preoperative chemoradiotherapy for oesophageal cancer: a systematic
review and meta-analysis. Gut 2004;53:925-30.[LinkOut]
- Vazquez-Sequeiros E, Wang L, Burgart L, Harmsen W, Zinsmeister A, Allen
M, et al. Occult lymph node metastases as a predictor of tumor relapse
in patients with node-negative esophageal carcinoma. Gastroenterology
2002;122:1815-21.[LinkOut]
- Sanghvi P, Choi M, Holland J, Thomas CR. Adjuvant (Postoperative)
Therapy. In: Blair JA, Thomas CR, Hunter JG, editors. Esophageal Cancer:
Principles and Practice. New York: Demos; 2008. p. 401-6.
- Xiao ZF, Yang ZY, Liang J, Miao YJ, Wang M, Yin WB, et al. Value of
radiotherapy after radical surgery for esophageal carcinoma: a report of 495
patients. Ann Thorac Surg 2003;75:331-6.[LinkOut]
- Pramesh CS, Mistry RC, Deshpande RK, Sharma S. Do we need more
trials of postoperative radiotherapy after esophagectomy? Ann Thorac Surg
2004;77:1878-9.[LinkOut]
- Xiao ZF, Yang ZY, Miao YJ, Wang LH, Yin WB, Gu XZ, et al. Influence of
number of metastatic lymph nodes on survival of curative resected thoracic
esophageal cancer patients and value of radiotherapy: report of 549 cases.
Int J Radiat Oncol Biol Phys 2005;62:82-90.[LinkOut]
- Schreiber D, Rineer J, Vongtama D, Wortham A, Han P, Schwartz D, et
al. Impact of postoperative radiation after esophagectomy for esophageal
cancer. J Thorac Oncol 2010;5:244-50.[LinkOut]
- Ténière P, Hay JM, Fingerhut A, Fagniez PL. Postoperative radiation
therapy does not increase survival after curative resection for squamous cell
carcinoma of the middle and lower esophagus as shown by a multicenter
controlled trial. French University Association for Surgical Research. Surg
Gynecol Obstet 1991;173:123-30.[LinkOut]
- Fok M, Sham JS, Choy D, Cheng SW, Wong J. Postoperative radiotherapy
for carcinoma of the esophagus: a prospective, randomized controlled
study. Surgery 1993;113:138-47.[LinkOut]
- Zieren HU, Müller JM, Jacobi CA, Pichlmaier H, Müller RP, Staar S.
Adjuvant postoperative radiation therapy after curative resection of
squamous cell carcinoma of the thoracic esophagus: a prospective
randomized study. World J Surg 1995;19:444-9.[LinkOut]
- Malthaner RA, Wong RK, Rumble RB, Zuraw L. Neoadjuvant or adjuvant
therapy for resectable esophageal cancer: a systematic review and metaanalysis.
BMC Med 2004;2:35.[LinkOut]
- A comparison of chemotherapy and radiotherapy as adjuvant treatment to
surgery for esophageal carcinoma. Japanese Esophageal Oncology Group.
Chest 1993;104:203-7.[LinkOut]
- Chen G, Wang Z, Liu XY, Liu FY. Adjuvant radiotherapy after modified
Ivor-Lewis esophagectomy: can it prevent lymph node recurrence of the
mid-thoracic esophageal carcinoma? Ann Thorac Surg 2009;87:1697-702.[LinkOut]
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC,
Stemmermann GN, et al. Chemoradiotherapy after surgery compared with
surgery alone for adenocarcinoma of the stomach or gastroesophageal
junction. N Engl J Med 2001;345:725-30.[LinkOut]
- Greene F, Page D, Fleming I, Fritz AG, Balch CM, Haller DG, et al.,
editors. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002.
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer
Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol
2010;17:1721-4.[LinkOut]
- Rice TW, Adelstein DJ, Chidel MA, Rybicki LA, DeCamp MM, Murthy
SC, et al. Benefit of postoperative adjuvant chemoradiotherapy in
locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg
2003;126:1590-6.[LinkOut]
- Bédard EL, Inculet RI, Malthaner RA, Brecevic E, Vincent M, Dar R. The
role of surgery and postoperative chemoradiation therapy in patients with
lymph node positive esophageal carcinoma. Cancer 2001;91:2423-30.[LinkOut]
- Liu HC, Hung SK, Huang CJ, Chen CC, Chen MJ, Chang CC, et al.
Esophagectomy for locally advanced esophageal cancer, followed by
chemoradiotherapy and adjuvant chemotherapy. World J Gastroenterol
2005;11:5367-72.[LinkOut]
- Dieleman EM, Senan S, Vincent A, Lagerwaard FJ, Slotman BJ, van
Sörnsen de Koste JR. Four-dimensional computed tomographic analysis
of esophageal mobility during normal respiration. Int J Radiat Oncol Biol
Phys 2007;67:775-80.[LinkOut]
- Gao XS, Qiao X, Wu F, Cao L, Meng X, Dong Z, et al. Pathological
analysis of clinical target volume margin for radiotherapy in patients with
esophageal and gastroesophageal junction carcinoma. Int J Radiat Oncol
Biol Phys 2007;67:389-96.[LinkOut]
- Qiao XY, Wang W, Zhou ZG, Gao XS, Chang JY. Comparison of efficacy of
regional and extensive clinical target volumes in postoperative radiotherapy
for esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys
2008;70:396-402.[LinkOut]
- Meier I, Merkel S, Papadopoulos T, Sauer R, Hohenberger W, Brunner TB.
Adenocarcinoma of the esophagogastric junction: the pattern of metastatic
lymph node dissemination as a rationale for elective lymphatic target
volume definition. Int J Radiat Oncol Biol Phys 2008;70:1408-17.[LinkOut]
- Nakamura T, Hatooka S, Kodaira T, Tachibana H, Tomita N, Nakahara
R, et al. Determination of the irradiation field for clinical T1-T3N0M0
thoracic/abdominal esophageal cancer based on the postoperative
pathological results. Jpn J Clin Oncol 2009;39:86-91.[LinkOut]
Cite this article as:
Jabbour S, Thomas C Jr. Radiation therapy in the postoperative management of esophageal cancers. J Gastrointest Oncol. 2010;1(2):102-111. DOI:10.3978/j.issn.2078-6891.2010.013
|