HER2 status in Barrett’s esophagus & esophageal cancer: a meta analysis
Introduction
The incidence of oesophageal adenocarcinoma (ADC) has increased more quickly than for any other malignancy in many western countries (1,2) and the rate of ADC is expected to rise in the coming decades (3). Barrett’s Esophagus (BE) is a major risk factor for the development of Esophageal Cancer (EC) (4-6). Understanding the role and prevalence of biomarkers such as human epidermal growth factor receptor 2 (HER2) in BE can possibly prevent the progression of this condition to its most lethal form, ADC, which is known for having an extremely poor prognosis, with an overall 5-year survival of around 10% (7) and potentially allow for early intervention for EC.
HER2 positivity status is thought to play a critical role in the development, progression and metastasis of many malignancies such as breast cancer & gastric cancer (8,9). HER2 is over-expressed by at least one fourth of human breast cancers and correlates with poor clinical outcome in women with node-positive and node-negative disease (10). HER2 targeted therapy (trastuzumab) has improved the outcomes of patients with breast cancer that over-expresses HER2 (11,12). A combination of the monoclonal antibody against HER2 (trastuzumab) with standard chemotherapy improved survival significantly in patients with HER2 positive advanced gastric cancer in the Trastuzumab for Gastric Cancer (ToGA) trial (13). However, the role of HER2 in the development and prognosis of BE & EC is yet to be clarified.
A meta-analysis of the prevalence of HER2 in both BE & EC has to date not been published. Our aim was to perform a meta-analysis combining the results of studies reporting HER2 status in BE & EC, and thus provide a quantitative estimate of the prevalence of HER2+ in BE & EC, and subsequently patient survival. We hypothesized that there will be an increased rate of HER2+ in patients with BE and EC. We also hypothesize that HER2+ will decrease survival time in subjects with EC.
Methods
Literature search strategy
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. A systematic search of the databases MEDLINE (from 1950), PubMed (from 1946), EMBASE (from 1949), PubMed (from 1950), and Current Contents Connect (from 1980) through to 2013, to identify relevant articles. The search used the terms ‘EC’ OR ‘BE’ AND ‘HER2’ OR ‘c-erbB2’, which were searched as text word and as exploded medical subject headings where possible. The reference lists of relevant articles were also searched for appropriate studies. No language restrictions were used in either the search or study selection. A search for unpublished literature was not performed.
Study selection
We included studies that met the following inclusion criteria: (I) HER2 positivity was measured in subjects with BE; (II) HER2 positivity was measured in subjects with EC; (III) Diagnostic method was reported; (IV) Prevalence of HER2 in BE or EC was reported. We excluded studies that did not meet the inclusion criteria.
Data extraction
The data extraction was performed using a standardized data extraction form, collecting information on the publication year, study design, number of cases, number of controls (if any), total sample size, temporal direction, population type, country, continent, mean age, number of adjusted variables, the risk estimates or data used to calculate the risk estimates, confidence intervals (CI) or data used to calculate CIs, the rate of HER2 expression & amplification. Quality of the studies was not assessed and authors were not contacted for missing data.
Statistical analysis
Pooled event rates (ER) and 95% confidence intervals were calculated for the prevalence of HER2 in subjects with BE or EC (14). We tested heterogeneity with Cochran’s Q statistic, with P<0.10 indicating heterogeneity, and quantified the degree of heterogeneity using the I2 statistic, which represents the percentage of the total variability across studies which is due to heterogeneity. I2 values of 25%, 50% and 75% corresponded to low, moderate and high degrees of heterogeneity respectively (15). The quantified publication bias using the Egger’s regression model (16), with the effect of bias assessed using the fail-safe number method. The fail-safe number was the number of studies that we would need to have missed for our observed result to be nullified to statistical non-significance at the P<0.05 level. Publication bias is generally regarded as a concern if the fail-safe number is less than 5n+10, with n being the number of studies included in the meta-analysis (17). All analyses were performed with Comprehensive Meta-analysis (version 2.0), Biostat, Englewood NJ (2005).
Results
Study characteristics
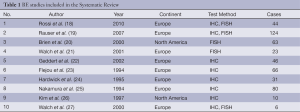
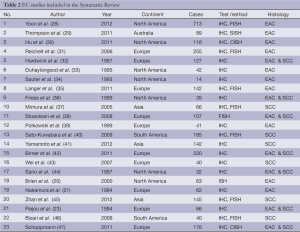
From 1,403 studies initially identified, 33 met our inclusion criteria (Figure 1). Selected characteristic of the included studies are presented in Tables 1,2. The studies represented a variety of geographical regions. Sample sizes ranged from 6 to 124 in BE studies and 14 to 713 in EC studies.
Full table
Full table
BE
Ten studies with 493 subjects in total were included in the meta-analysis for BE. The mean age was 63.85. The average percentage of males with Barrett’s associated ADC was 85.06%. The average percentage of females with BE was 12.82%. Only two studies reported percentage of HER2 positivity among male & females.
BE & IHC
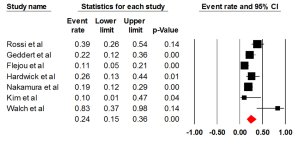
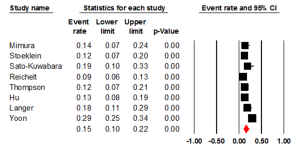
Seven studies examined the status of HER2 through IHC, with an ER of 0.24 (95% CI: 0.15-0.36) (Figure 2). There was statically significant heterogeneity (I2= 69.14%, P=0.006). The Egger test for publication bias was not significant (P=0.43). A regional comparison was not carried out for BE as 6 out of 7 studies were conducted in Europe.
BE & FISH
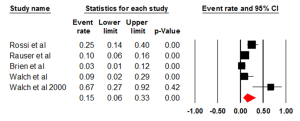
Five studies evaluated the prevalence of HER2 positivity through FISH, with an ER of 0.15 (95% CI: 0.06-0.33) (Figure 3). There was statically significant heterogeneity (I2=80.00%, P<0.001). The Egger test for publication bias was not significant (P=0.89). A regional comparison was not carried out for BE as 4 out of 5 studies were conducted in Europe.
EC
Twenty three studies with 3,032 were included in the meta-analysis for EC and HER2. The mean age was 63. The average percentage of males with EC was 85.0%, of these an average of 25.14% were HER2 positive. The average percentage of females with EC was 15.0% of these an average of 28.14% were HER2 positive.
EC & IHC
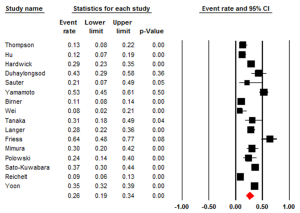
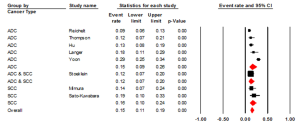
Studies that examined HER2 positivity through IHC had an ER of 0.26 (95% CI: 0.19-0.34) (Figure 4). There was statistically significant heterogeneity (I2=92.45%, P<0.001). The Egger test for publication bias was not significant (P=0.25). The studies evaluating HER2+ in ADC had an ER of 0.21 (95% CI: 0.14-0.32, P<0.001). Studies that examined HER2 in squamous cell carcinoma (SCC) had an ER of 0.32 (95% CI: 0.20-0.48). The studies that investigated HER2+ in both ADC & SCC had an ER of 0.30 (95% CI: 0.13-0.55). All three groups, ADC, SCC and the combination had a statistically significant heterogeneity (P<0.001), I2=91.67%, I2=88.08 and I2=95.03 respectively. We also evaluated the regional influence of HER2+ in EC. It was found that Asia had an ER of 0.42 (95% CI: 0.22-0.64) with a statistically significant heterogeneity (I2=88.82%, P=0.003). Europe had an ER of 0.17 (95% CI: 0.10-0.27) with a statistically significant heterogeneity (I2=90.79%, P=0.001). North America had an ER of 0.33 (95% CI: 0.21-0.48). There was statistically significant heterogeneity (I2=86.93%, P<0.001).
EC & ISH
We found an ER of 0.15 (95% CI: 0.10-0.22) (Figure 5). There was statistically significant heterogeneity (I2=86.01%, P<0.001). The Egger test for publication bias was not significant (P=0.43). The studies were also evaluated by cancer types (ADC & SCC) (Figure 6). We found an ER of 0.15 (95% CI: 0.09-0.26) for ADC, with a statistically significant heterogeneity (I2=91.13%, P<0.001). The ER for SCC was 0.16 (95% CI: 0.10-0.24), with a statistically non-significant heterogeneity (I2=0%, P=0.43). We also evaluated the regional influence of HER2+ in EC. It was found that Europe had an ER of 0.12 (95% CI: 0.08-0.19). There was statistically non-significant heterogeneity (I2=60.17%, P=0.08). North America had an ER of 0.20 (95% CI: 0.08-0.41). There was statistically significant heterogeneity (I2= 93.83%, P<0.001).
EC & survival
The pooled HR is 1.45 (95% CI: 0.85-2.48). It was not statistically significantly (P=0.17). Between groups HER2+ & HER2-, a difference of 7 months was noted (95% CI: 6-20 months). This was not statistically significant (P=0.29).
Discussion
Our meta-analysis shows that there is a high prevalence rate of HER2+ in both BE and EC populations, 24% and 26%, respectively. The prevalence rate of HER2+ in EC and BE is higher than that of Breast Cancer (12,48). The ratio between male and females in the studies was 5:1 in both BE and EC subjects. From EC studies it was shown that although the proportion of women diagnosed with EC was lower than males, the prevalence of HER2+ was slightly higher. Men had an event rate of 25.14%, while women were 28.14%. On the contrary, analysis of the two studies that had reported HER2+ percentage among males and females in BE studies showed that the prevalence of HER2+ among male was almost double that of women. Both BE and EC studies have shown that Stage III had the highest percentage of patients. The low level of HER2+ in Stage I and II can be explained by the late diagnosis of the disease. The significance of tumour staging in HER2+ is still not clear.
Studies assessing BE show wide variation in terms of HER2+. Almost half the studies classified the patient groups as having either low or high grade dysplasia, while other studies classified patients as having Barrett’s associated ADC. These studies have the potential of misclassification bias and increased heterogeneity due to the mixing of these two groups. Further studies of pure Barrett’s oesophagus patients are required. The effect of reflux disease on the HER2+ rate is unknown as no studies have specifically addressed this patient group.
A larger proportion of the included BE studies analysed HER2 status using IHC while a very small number have used FISH. This validates the results as the diagnostic method is of the same nature in the included studies. Another consistent factor noticed in the BE studies was the regional variation. The majority of the studies have conducted the analysis in European patients/region, which once again provides accuracy in analysing these data as one. The BE sample size is relatively low, this may decrease the quality and power of the BE analysis. Our findings suggest that the investigation of HER 2 might be beneficial in characterizing the progression from BO to dysplasia and ADC. These potential markers might also contribute to deciding alternative therapeutic methods, as advised by some preliminary data (50).
The prevalence rate of HER2+ among patients with SCC was significantly higher than that of ADC. When comparing studies that have included both ADC and SCC, the reason for this difference of HER2+ between ADC and SCC is unclear. Hardwick et al. (32) have analysed HER2+ among ADC and SCC separately and have shown that SCC has a higher HER2+ prevalence than ADC. On the other hand, Birner et al. (42) have shown that ADC has a higher HER2+ rate than SCC. The two remaining studies Stoecklein et al. (38) and Friess et al. (36) have combined the prevalence rate of HER2+ among ADC and SCC and therefore prevalence rates between the two groups was not defined. The meta-analysis has shown that an event rate of HER2+ in EC was highest in Asian regions. This is likely due to the fact that Asian regions, especially China have the highest incidence of SCC in the world (51,52). This increased rate of incidence could be due to risk factors such as genetic predisposition (51), high concentrations of nitrate nitrogen in drinking water (53) and other water resources (54).
The survival analysis among the EC studies concluded that subject who are HER2+ have an average decreased survival rate of 7 months. Although the accumulated results conclude that HER2+ leads to poor prognosis compared to HER2-, a handful of the studies that were included such as Duhaylongsod et al. (33) and Yoon et al. (28) have stated that HER2+ improves survival compared to HER2-. Four studies (29,30,32,36) have concluded that HER2+ does not make a difference in survival rate, while six studies (31,35,37-40) report that HER2+ decreases the survival rate. The discrepancies among results can be due to factors such as, patient definition, diagnostic methods, and classification of HER2+. It has been suggested that poorer survival in HER2-positive patients with squamous cell carcinoma could be due to increased resistance to radiation therapy (55) and cisplatinum-based chemotherapy (56). Moreover, the addition of trastuzumab in head and neck squamous cell carcinoma cell lines seemed to enhance the effect of irradiation (57).
The statistically significant heterogeneity and publication bias amongst the included studies may be due to several factors. There is a slight variation in the patient eligibility for each study. These differences in patient definition can lead to potential bias and could drive the analysis in one direction. Excluding studies that appear to be outliers may have potentially reduced heterogeneity. Due to the limited number of studies available in this area, excluding these studies will reduce sample size and consequently increase heterogeneity once again.
Similarly, the classification system used between each study for HER2+ varies. Studies such as Hu et al. (30), Reichelt et al. (31), Wei et al. (43) and Sato-Kuwabara et al. (40) have classified HER2+ as IHC ≥2 while Mimura et al. (37) have drawn the line at IHC ≥1, and Langer et al. (35) have classified HER2+ as IHC 3+. Similarly with FISH, Langer et al. (35), Mimura et al. (37), Thompson et al. (29) and Hu et al. (30) have classified HER2+ as FISH 2+, while Sato-Kuwabara et al. (40) have classified HER2+ as FISH 3+. A standardized classification system is required in order to determine the full potential of HER2+ in EC. Misclassification of IHC results will consequently affect results of FISH. There was a variation in event rate between the diagnostic methods. The ER of HER2+ was high through IHC, in comparison to the ER of HER2+ through FISH (for both BE & EC). Ahmed et al. (58) has stated that in the case of breast cancer results of IHC and FISH require a minimum of 95% concordance, which we have not seen in the current study. Barrett et al. (59) has highlighted that IHC 2+ weak positive are often not accompanied by a FISH positive or represent gene amplification in breast cancer tissues. The HercepTest™ is considered valid for the identification of HER2+ in the case of gastric cancer (60), no classification system has been implemented for EC. The accuracy of the IHC HER2+ results is vital in determining the FISH status. The validity of the results can also be questioned by the diagnostic method each study has used.
Studies such as Reichelt et al. (31) provided strong clinical and experimental data and by collaborating these data they have provided survival outcomes of patients, which was vital in the survival analysis. This study also had strong FISH and IHC concordance. The studies that have studied one histology of EC (ADC or SCC) have a higher quality of data in comparison to studies that have combined these data. This is reflective in the homogeneity of the study sample.
Langer et al. (35) has stated that the correlation between the biomarker and increase mortality can only be demonstrated through 3D in situ hybridization. This raises the question of validity among all other studies that have not carried out this technique but have completed a survival analysis. Studies published prior to 2000 have examined molecular markers such as c-erb2 and p53, while studies post 2000 have focused on HER2. There is evidently a variation in prognostic factors. While Yoon et al. (28) has reported that two pathologists were used to examine HER2+, many other studies have failed to mention methods used to analyse HER2+.
The Mayo Clinic (28) has so far published the largest cohort evaluating the relationship between HER2/ErbB2 expression and oesophageal adenocarcinomas out of the 713 patients (17%) of EACs were HER2+, with strong agreement between HER2 amplification and expression (k=0.83). HER2+ was significantly associated with lower tumour grade, less invasiveness, fewer malignant nodes, and the presence of adjacent BE. EACs with BE had higher odds of HER2 positivity than EACs without BE, independent of pathologic features [OR=1.8 (95% CI: 1.1-2.8)]. Among all cases, HER2 positivity was significantly associated with disease-specific survival (DSS) in a manner that differed by the presence or absence of BE (Pinteraction=0.0047). In EACs with BE, HER2 positivity was significantly associated with improved DSS [HR=0.54 (95% CI: 0.35-0.84), P=0.0065] and overall survival (P=0.0022) independent of pathologic features, but was not prognostic among EACs without BE.
In the recently published ToGA trial (13), which was the first randomized, controlled, Phase III trial for gauging the effectiveness of trastuzumab in gastric cancer, A total of 594 with locally advanced or metastatic HER2-overexpressing adenocarcinoma of the stomach or gastroesophageal junction (GEJ) were randomized to receive trastuzumab plus chemotherapy or chemotherapy alone. Twenty-two per cent of patients out of more than 3,800 cases screened in 24 countries showed HER2 expression, with a good concordance rate between IHC staining and FISH. The tumours were confirmed to be either HER2 gene amplified by FISH or protein overexpressing via IHC. The patients were included in the study only if the tumour was scored as 3+ on IHC or if it was 2+ on IHC and FISH positive
Among the patients that entered the study, 82% had primary gastric cancer and 18% had primary GEJ adenocarcinoma. Ninety-seven per cent had metastatic disease. The median age was 60 years (range, 21-83 years) and 76% were male. Previous therapies included gastrectomy (23%), previous neoadjuvant and/or adjuvant therapy (7%) and previous radiotherapy (2%). Trastuzumab was administered at an initial dose of 8 mg/kg intravenously followed by 6 mg/kg every 3 weeks until disease progression or significant toxicity. The chemotherapy regimen comprised of cisplatin 80 mg/m2 intravenously every 3 weeks for six cycles and a fluoropyrimidine (either capecitabine 1,000 mg/m2 orally twice daily for 14 days or 5-fluorouracil 800 mg/m2/day continuous intravenous infusion for 5 days every 3 weeks for six cycles).
The trial was sealed after the second interim analysis when 167 deaths had occurred on the trastuzumab arm and 184 deaths on the control arm. In the final analysis, the median survival was 13.8 months in patients allocated to trastuzumab plus chemotherapy compared with 11.1 months in chemotherapy group alone (P=0.0046). Overall tumour response, complete or partial, was significantly increased (47% vs. 35%) in trastuzumab plus chemotherapy arm versus chemotherapy alone. The hazard ratio (HR) was 0.74 (95% CI: 0.60-0.91; P=0.0036, two sided) in favour of the trastuzumab arm. Exploratory survival analyses in subgroups defined by IHC testing indicated that trastuzumab was most effective in prolonging survival in the IHC 3+ tumours and less effective in IHC 2+ tumours. However, the final exploratory survival analyses included only the HER2/neu FISH positive patients.
In October 2010, the FDA granted approval for trastuzumab in combination with cisplatin and a fluoropyrimidine (capecitabine or 5-fluorouracil) for the treatment of patients with HER2-overexpressing metastatic gastric or GEJ adenocarcinoma who have not received previous treatment for metastatic disease (13). Several ongoing trials have the goal of evaluating trastuzumab in oesophagogastric and/or gastric cancer in the first line in combination with chemotherapy or as a salvage agent in recurrent cancer.
In conclusion, it was seen that HER2+ prevalence in both BE and EC was relatively high with approximately a forth of patients indicating HER2+. HER2+ in EC has been shown to decrease survival. HER2+ targeted therapy for eligible patients should be considered and carried out in a clinical trial. Further studies looking at HER2+ effect on survival should also be carried out with all relevant diagnostic methods and classification systems used.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lepage C, Rachet B, Jooste V, et al. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol 2008;103:2694-9. [PubMed]
- Bremholm L, Funch-Jensen P, Eriksen J, et al. Barrett’s esophagus. Diagnosis, follow-up and treatment. Dan Med J 2012;59:C4499. [PubMed]
- Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol 2012;23:3155-62. [PubMed]
- Wang S, Zheng G, Chen L, et al. Effect of HER-2/neu over-expression on prognosis in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev 2011;12:1417-23. [PubMed]
- Picardo SL, Maher SG, O’Sullivan JN, et al. Barrett’s to oesophageal cancer sequence: a model of inflammatory-driven upper gastrointestinal cancer. Dig Surg 2012;29:251-60. [PubMed]
- Mahzouni P, Movahedipour M. An immunohistochemical study of HER2 expression in meningioma and its correlation with tumor grade. Pathol Res Pract 2012;208:221-4. [PubMed]
- Agarwal A, Polineni R, Hussein Z, et al. Role of epigenetic alterations in the pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma. Int J Clin Exp Pathol 2012;5:382-96. [PubMed]
- Yu GZ, Chen Y, Wang JJ, et al. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol 2009;135:1331-9. [PubMed]
- Liang Z, Zeng X, Gao J, et al. Analysis of EGFR, HER2, and TOP2A gene status and chromosomal polysomy in gastric adenocarcinoma from Chinese patients. BMC Cancer 2008;8:363. [PubMed]
- Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17:2639-48. [PubMed]
- Murphy CG, Morris PG. Recent advances in novel targeted therapies for HER2-positive breast cancer. Anticancer Drugs 2012;23:765-76. [PubMed]
- Guiu S, Arnould L, Bonnetain F, et al. Pathological response and survival after neoadjuvant therapy for breast cancer: A 30-year study. Breast 2013;22:301-8. [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Orwin RG. A fail-safe N for effect size in meta-analysis. Journal of Educational Statistics 1983;8:157-9.
- Rossi E, Villanacci V, Bassotti G, et al. TOPOIIalpha and HER-2/neu overexpression/amplification in Barrett’s oesophagus, dysplasia and adenocarcinoma. Histopathology 2010;57:81-9. [PubMed]
- Rauser S, Weis R, Braselmann H, et al. Significance of HER2 low-level copy gain in Barrett’s cancer: implications for fluorescence in situ hybridization testing in tissues. Clin Cancer Res 2007;13:5115-23. [PubMed]
- Brien TP, Odze RD, Sheehan CE, et al. HER-2/neu gene amplification by FISH predicts poor survival in Barrett’s esophagus-associated adenocarcinoma. Hum Pathol 2000;31:35-9. [PubMed]
- Walch A, Specht K, Bink K, et al. Her-2/neu gene amplification, elevated mRNA expression, and protein overexpression in the metaplasia-dysplasia-adenocarcinoma sequence of Barrett’s esophagus. Lab Invest 2001;81:791-801. [PubMed]
- Geddert H, Zeriouh M, Wolter M, et al. Gene amplification and protein overexpression of c-erb-b2 in Barrett carcinoma and its precursor lesions. Am J Clin Pathol 2002;118:60-6. [PubMed]
- Fléjou JF, Paraf F, Muzeau F, et al. Expression of c-erbB-2 oncogene product in Barrett’s adenocarcinoma: pathological and prognostic correlations. J Clin Pathol 1994;47:23-6. [PubMed]
- Hardwick RH, Shepherd NA, Moorghen M, et al. c-erbB-2 overexpression in the dysplasia/carcinoma sequence of Barrett’s oesophagus. J Clin Pathol 1995;48:129-32. [PubMed]
- Nakamura T, Nekarda H, Hoelscher AH, et al. Prognostic value of DNA ploidy and c-erbB-2 oncoprotein overexpression in adenocarcinoma of Barrett’s esophagus. Cancer 1994;73:1785-94. [PubMed]
- Kim R, Clarke MR, Melhem MF, et al. Expression of p53, PCNA, and C-erbB-2 in Barrett’s metaplasia and adenocarcinoma. Dig Dis Sci 1997;42:2453-62. [PubMed]
- Walch A, Bink K, Hutzler P, et al. HER-2/neu gene amplification by FISH predicts poor survival in Barrett’s esophagus-associated adenocarcinoma. Hum Pathol 2000;31:1332-4. [PubMed]
- Yoon HH, Shi Q, Sukov WR, et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res 2012;18:546-54. [PubMed]
- Thompson SK, Sullivan TR, Davies R, et al. Her-2/neu gene amplification in esophageal adenocarcinoma and its influence on survival. Ann Surg Oncol 2011;18:2010-7. [PubMed]
- Hu Y, Bandla S, Godfrey TE, et al. HER2 amplification, overexpression and score criteria in esophageal adenocarcinoma. Mod Pathol 2011;24:899-907. [PubMed]
- Reichelt U, Duesedau P, Tsourlakis M, et al. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol 2007;20:120-9. [PubMed]
- Hardwick RH, Barham CP, Ozua P, et al. Immunohistochemical detection of p53 and c-erbB-2 in oesophageal carcinoma; no correlation with prognosis. Eur J Surg Oncol 1997;23:30-5. [PubMed]
- Duhaylongsod FG, Gottfried MR, Iglehart JD, et al. The significance of c-erb B-2 and p53 immunoreactivity in patients with adenocarcinoma of the esophagus. Ann Surg 1995;221:677-83. [PubMed]
- Sauter ER, Keller SM, Erner S, et al. HER-2/neu: a differentiation marker in adenocarcinoma of the esophagus. Cancer Lett 1993;75:41-4. [PubMed]
- Langer R, Rauser S, Feith M, et al. Assessment of ErbB2 (Her2) in oesophageal adenocarcinomas: summary of a revised immunohistochemical evaluation system, bright field double in situ hybridisation and fluorescence in situ hybridisation. Mod Pathol 2011;24:908-16. [PubMed]
- Friess H, Fukuda A, Tang WH, et al. Concomitant analysis of the epidermal growth factor receptor family in esophageal cancer: overexpression of epidermal growth factor receptor mRNA but not of c-erbB-2 and c-erbB-3. World J Surg 1999;23:1010-8. [PubMed]
- Mimura K, Kono K, Hanawa M, et al. Frequencies of HER-2/neu expression and gene amplification in patients with oesophageal squamous cell carcinoma. Br J Cancer 2005;92:1253-60. [PubMed]
- Stoecklein NH, Hosch SB, Bezler M, et al. Direct genetic analysis of single disseminated cancer cells for prediction of outcome and therapy selection in esophageal cancer. Cancer Cell 2008;13:441-53. [PubMed]
- Polkowski W, van Sandick JW, Offerhaus GJ, et al. Prognostic value of Lauren classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol 1999;6:290-7. [PubMed]
- Sato-Kuwabara Y, Neves JI, Fregnani JH, et al. Evaluation of gene amplification and protein expression of HER-2/neu in esophageal squamous cell carcinoma using Fluorescence in situ Hybridization (FISH) and immunohistochemistry. BMC Cancer 2009;9:6. [PubMed]
- Yamamoto Y, Yamai H, Seike J, et al. Prognosis of esophageal squamous cell carcinoma in patients positive for human epidermal growth factor receptor family can be improved by initial chemotherapy with docetaxel, fluorouracil, and cisplatin. Ann Surg Oncol 2012;19:757-65. [PubMed]
- Birner P, Jesch B, Friedrich J, et al. Carbonic anhydrase IX overexpression is associated with diminished prognosis in esophageal cancer and correlates with Her-2 expression. Ann Surg Oncol 2011;18:3330-7. [PubMed]
- Wei Q, Chen L, Sheng L, et al. EGFR, HER2 and HER3 expression in esophageal primary tumours and corresponding metastases. Int J Oncol 2007;31:493-9. [PubMed]
- Sano A, Kato H, Sakurai S, et al. CD24 expression is a novel prognostic factor in esophageal squamous cell carcinoma. Ann Surg Oncol 2009;16:506-14. [PubMed]
- Zhan N, Dong WG, Tang YF, et al. Analysis of HER2 gene amplification and protein expression in esophageal squamous cell carcinoma. Med Oncol 2012;29:933-40. [PubMed]
- Bizari L, Borim AA, Leite KR, et al. Alterations of the CCND1 and HER-2/neu (ERBB2) proteins in esophageal and gastric cancers. Cancer Genet Cytogenet 2006;165:41-50. [PubMed]
- Schoppmann SF, Jesch B, Zacherl J, et al. HER-2 status in primary oesophageal cancer, lymph nodes and distant metastases. Br J Surg 2011;98:1408-13. [PubMed]
- Engelstaedter V, Schiffers J, Kahlert S, et al. Her-2/neu and topoisomerase IIalpha in advanced breast cancer: a comprehensive FISH analysis of 245 cases. Diagn Mol Pathol 2012;21:77-83. [PubMed]
- Ryu DW, Lee CH. Impact of Serum HER2 Levels on Survival and Its Correlation with Clinicopathological Parameters in Women with Breast Cancer. J Breast Cancer 2012;15:71-8. [PubMed]
- Villanacci V, Rossi E, Grisanti S, et al. Targeted therapy with trastuzumab in dysplasia and adenocarcinoma arising in Barrett’s esophagus: a translational approach. Minerva Gastroenterol Dietol 2008;54:347-53. [PubMed]
- Zhang H, Chen Z, Cheng J, et al. The high incidence of esophageal cancer in parts of China may result primarily from genetic rather than environmental factors. Dis Esophagus 2010;23:392-7. [PubMed]
- Zheng S, Vuitton L, Sheyhidin I, et al. Northwestern China: a place to learn more on oesophageal cancer. Part one: behavioural and environmental risk factors. Eur J Gastroenterol Hepatol 2010;22:917-25. [PubMed]
- Zhang N, Yu C, Wen D, et al. Association of nitrogen compounds in drinking water with incidence of esophageal squamous cell carcinoma in Shexian, China. Tohoku J Exp Med 2012;226:11-7. [PubMed]
- He Z, Zhao Y, Guo C, et al. Prevalence and risk factors for esophageal squamous cell cancer and precursor lesions in Anyang, China: a population-based endoscopic survey. Br J Cancer 2010;103:1085-8. [PubMed]
- Dreilich M, Wanders A, Brattstrom D, et al. HER-2 overexpression (3+) in patients with squamous cell esophageal carcinoma correlates with poorer survival. Dis Esophagus 2006;19:224-31. [PubMed]
- Akamatsu M, Matsumoto T, Oka K, et al. c-erbB-2 oncoprotein expression related to chemoradioresistance in esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2003;57:1323-7. [PubMed]
- Uno M, Otsuki T, Kurebayashi J, et al. Anti-HER2-antibody enhances irradiation-induced growth inhibition in head and neck carcinoma. Int J Cancer 2001;94:474-9. [PubMed]
- Ahmed SS, Iqbal J, Thike AA, et al. HER2/neu revisited: quality and interpretive issues. J Clin Pathol 2011;64:120-4. [PubMed]
- Barrett C, Magee H, O’Toole D, et al. Amplification of the HER2 gene in breast cancers testing 2+ weak positive by HercepTest immunohistochemistry: false-positive or false-negative immunohistochemistry? J Clin Pathol 2007;60:690-3. [PubMed]
- Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797-805. [PubMed]