The role of induction chemotherapy + chemoradiotherapy in localised pancreatic cancer: initial experience in Scotland
Introduction
Despite being a relatively rare cancer, pancreatic ductal adenocarcinoma (PDAC) has recently become the third highest cause of death in females worldwide, having overtaken the much more prevalent breast cancer (1). It is also the 4th commonest cause of death in men and little impact has been made on improving survival over several decades (1). In the UK in 2010, 8,463 people were diagnosed with pancreatic cancer and there were 7,901 deaths (1). Five year overall survival remains extremely low at approximately 5% (1).
Surgery is possible in 10–20% of cases and with the evidence supporting a survival advantage with adjuvant chemotherapy—this is now the accepted standard of care. In an analysis of data from the ESPAC 3 (2) study, completion of six cycles of adjuvant chemotherapy was associated with doubling of median survival from 14 to 28 months but unfortunately many patients fail to complete or even receive adjuvant therapy (2). In Scotland, an audit of treatment received for pancreatic cancer found that only 55% of patients undergoing resection for pancreatic cancer received adjuvant therapy (3). In ESPAC 3, where patients were already sub-selected as suitable to be considered for chemotherapy, only 68% of patients completed all six cycles (2).
Surgical resection is associated with positive resection margins in the majority of patients, and it is questionable whether primary resection is the appropriate approach in this group, where long term survival is rare.
Prior to 2011, the absence of effective chemotherapy, with the possibility of a meaningful response, was a major barrier to the use of neo-adjuvant chemotherapy in pancreatic cancer. Response rates of only 3–9% were reported with gemcitabine or gemcitabine combinations and experience with other drug combinations was limited (4,5). The advent of more effective chemotherapy, with FOLFIRINOX in metastatic pancreatic cancer, signalled a change in direction. In this study, not only was meaningful improvement in survival achieved, radiological response was seen in 30% of cases improvement in survival (11.1 vs. 6.8 months, P<0.001) (6).
Since then, there has been increasing interest in the use of FOLFIRINOX and other drug combinations in the neo-adjuvant setting, primarily for patients with borderline or irresectable disease where down-staging is desirable. There has also been some early experience with FOLFIRINOX in resectable pancreatic cancer, albeit in highly selected groups (7-9).
FOLFIRINOX is perceived as a toxic regimen. It was not widely adopted in the UK (and is still not used in many centres) and we were therefore keen to record toxicity in the non-metastatic group. This also provided an opportunity to explore efficacy in the LAPC group as the original clinical trial had been in metastatic patients only (6).
In 2012, following discussions in our MDT, we devised a protocol utilising FOLFIRINOX (FFX) in the non-metastatic pancreatic cancer setting. The primary aim of this study was to evaluate the effect on overall survival, radiological response, pathological response and to define the tolerability of this approach both from toxicity from chemotherapy and surgical morbidity. For patients who could not be treated with FFX, but were fit for combination chemotherapy, we offered Gemcitabine Capecitabine (GemCap) as induction chemotherapy and we collected the same data for these patients.
Methods
This was a retrospective analysis of a prospectively maintained database. Patients from 2012 to 2015 referred to the West of Scotland Regional Hepato-Pancreatic Biliary (HPB) MDT were included in this cohort. Prior to 2012, patients with resectable disease were treated with surgical resection followed, where possible, by adjuvant gemcitabine chemotherapy. Patients with localized borderline resectable or irresectable tumours were managed by gemcitabine chemotherapy.
Following 2012, patients with localized pancreatic cancer were stratified into four groups as detailed below. Our staging algorithm included, CT scan of chest, abdomen and pelvis within 6 weeks of surgery. In 2013, for patients without liver metastases on CT, we introduced an MRI liver with gadolinium contrast. This time period also spanned recruitment to the PET PANC (10) study where patients were also given PET CT as part of a prospective UK study. Endoscopic ultrasound was used for clarification of distant lymph node metastases and for tissue diagnosis in the majority of cases.
When staging was complete all patients were discussed at the regional MDT, classified according to resectability criteria and a treatment plan agreed. Where patients were deemed surgically resectable (A or B by the definitions in Table 1), formal cardiovascular fitness testing (CPET) was carried out. Patients managed by a “surgery first” approach are excluded from the current analysis. These represented a heterogeneous group with either tumours staged as “A”, tumours thought to arise in IPMN or patients in whom a tissue diagnosis was not obtained despite EUS FNA/FNB.

Full table
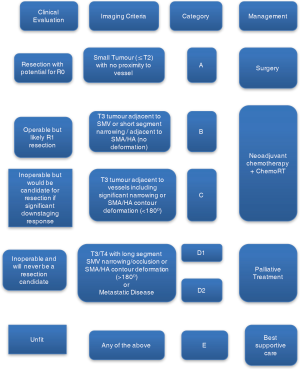
Our criteria for resectability are those of the NCCN guidelines (13):
- No distant metastases;
- Venous involvement of the SMV or PV with distortion or narrowing of the vein or occlusion of the vein widths suitable vessel proximal and distal, allowing safe resection and reconstruction;
- Gastro-duodenal artery encasement up to hepatic artery with either short segment encasement or direct abutment of the hepatic artery, without extension to the celiac axis;
- Tumour abutment of the SMA not to exceed greater than 180° of the circumference of the vessel wall.
Locally we have adapted these guidelines to assist in our regional MDT decision-making process (Figure 1).
Chemotherapy protocol (2012–2015)
Fit patients under 70 years of age were offered FFX. In patients deemed unfit for triplet therapy or over 70 years of age Gemcitabine/Capecitabine (GemCap) (11) was used (doses outlined in Table 1). A re-staging CT Chest Abdomen and Pelvis scan was performed to assess tumour response and resectability after 3 months of chemotherapy (either 6 cycles of FOLFIRINOX or 3 cycles of GemCap). At this juncture, the multidisciplinary team assessed the radiological response to treatment.
Chemoradiotherapy
Patients who had stable disease or better on a CT Chest Abdomen Pelvis approximately 4 weeks after completion of chemotherapy were considered for chemoradiotherapy (CRT). This comprised Volumetric Modulated Arc Therapy delivering 50.4 Gy in 28 fractions over 5.5 weeks with concurrent Capecitabine as per the SCALOP trial (11).Subsequently, if patients were considered potentially resectable, surgical exploration was undertaken. Two radiologists evaluated radiological response to chemotherapy prospectively as part of the multidisciplinary assessment.
We predicted from other retrospective series and also from peri-operative regimens in other tumour types—a progression rate of approximately 30% (12,14,15), and for these patients, second line chemotherapy, a clinical trial or best supportive care were considered, as appropriate.
Surgical resection
Where appropriate, patients underwent surgical resection in the West of Scotland Pancreatic Unit, Glasgow Royal Infirmary, and Glasgow, UK. Classical or pylorus-preserving pancreaticoduodenectomy, total or distal pancreatectomy, were performed by four surgeons (CRC, CJM, EJD and DKC). Surgical approach was unaltered through the study period. Briefly, following full laparotomy the right colon was fully mobilized and rotated to allow exposure of the intrapancreatic superior mesenteric vein. The duodenum was Kocherised clearing retroperitoneal and aortocaval lymphatics en bloc. The common hepatic duct was transected just below the portal confluence and the common hepatic artery cleared of lymphatics to its origin at the coeliac axis. Patients were usually managed by an “artery first” approach with complete exposure of the superior mesenteric artery and division of the superior and inferior pancreaticoduodenal branches before pancreatic and either duodenal or gastric transection. Following transection of the first jejunal loop and division of the mesentery, the duodenum and proximal jejunum were rotated posteriorly and dissection from the superior mesenteric/portal vein completed. Venous involvement was managed by vein resection and either primary repair or autologous vein graft as appropriate. Reconstruction was by single loop duodeno/gastro-jejunostomy, pancreaticojejunostomy or pancreaticogastrostomy and hepaticojejunostomy. Post-operative management was defined by an ERAS protocol.
Adjuvant chemotherapy protocol (2009–2012)
Following publication of the ESPAC 3 study (2), like many centres, the West of Scotland HPB Unit, adopted Gemcitabine 1,000 mg/m2 D1, 8, 15 q28 as its adjuvant regimen of choice.
Pathology assessment
During the study period, resection specimens were assessed by two specialist gastrointestinal pathologists with a specialist interest in pancreatic disease. Microscopic pathological assessment and reporting included: maximum tumour diameter and extent and location of local spread, tumor grade, perineural, venous, and lymphatic invasion, total of lymph nodes examined and number positive.
For this study, tumor grade was categorized as either high (for poorly differentiated tumors) or low (for moderately and well-differentiated tumors). On receipt of the specimen, the four pancreatic margins (pancreatic transection, medial, posterior and anterior surface) were identified and inked with different colours. Full details of the pathological assessment have been detailed previously (16).
Current guidelines define margin positivity as the presence of tumor at within 1 mm of a margin when assessed by microscopy of a hematoxylin-and-eosinophil stained slide.
Toxicity assessment
Toxicity was graded according to the CTC version 4 (17). All postoperative complications were prospectively recorded in a surgical outcomes database and graded through detailed weekly consensus discussion by the four operating pancreatic surgeons according to the International Study Group of Pancreatic Surgery (ISGPS) classifications and the Clavien-Dindo classification (18).
Statistics
All statistical testing was conducted at the 5% level so 95% confidence intervals (CI) are reported throughout. Unless otherwise stated, medians and interquartile range (IQR) are used. The survival time defined as the number of months from study entry until death or if alive at follow-up date, was calculated. Univariate survival analysis was carried out using Kaplan-Meier method and the log rank test. Survival analysis was carried out using the Cox’s proportional-hazards model and hazard ratios (HR) were calculated. Multivariate survival analysis was performed using a stepwise backward procedure to derive a final model of the variables that had a significant independent relationship with survival. To remove a variable from the multivariate model, the corresponding P value had to be >0.20.
Statistical analyses were performed using SPSS v21.0 (SPSS Inc., Chicago, IL).
Results
Baseline characteristics
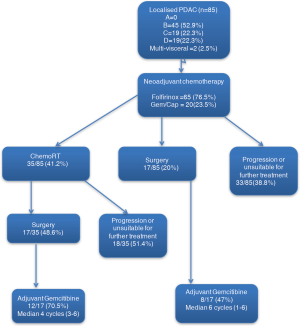
In total 85 patients were included in the study. The patients are shown in the consort diagram below (see Figure 2).
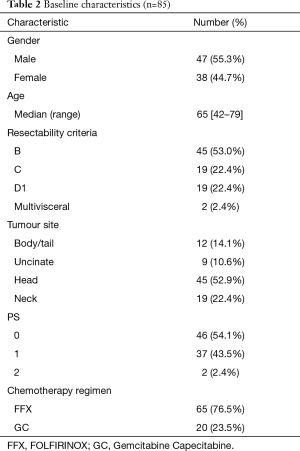
The median age was 65 (range 42–79) years and the majority were male with good performance status, and as per our local protocol were offered FFX chemotherapy as their induction chemotherapy regimen. Baseline characteristics are described in Table 2 below.
Full table
Toxicity in all non-metastatic patients (N=85)
FOLFIRINOX group
A total of 65 patients underwent chemotherapy with FFX. Table 3 outlines the patient characteristics. Thirty three (50.8%) completed 6 cycles as planned, 19 (29.2%) stopped chemotherapy early and 13 (20%) underwent further cycles (up to a maximum of 12). Fifteen (23.1%) patients started with a dose reduction. In total 40 (61.5%) required a dose reduction during their treatment. The majority of patients had at least one treatment delay (43, 66.1%) with 3 patients requiring their treatment to be stopped. All patients were treated with prophylactic Granulocyte Colony Stimulating Factor (GCSF day 5–10 sc 30,000 iU).
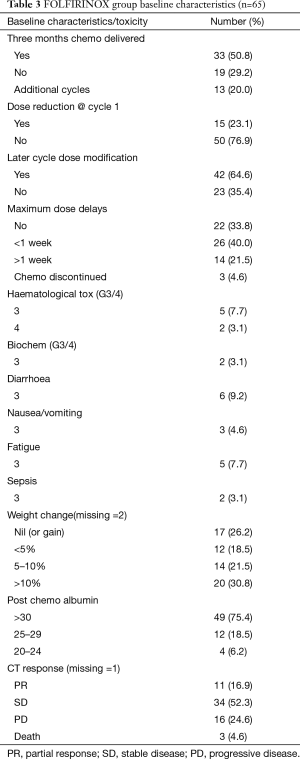
Full table
FFX was well tolerated. The commonest were haematological and gastrointestinal with 2 patients having febrile neutropenia (3.1%). Notably 20 (30.8%) of patients had weight loss of greater than 10% of baseline body weight. Of the 3 deaths 2 were attributed to pneumonia and sepsis in patients with pre-existing comorbidity while the other was due to rapid progression of cancer.
Gem/Cap group
Table 4 outlines baseline characteristics of group of patients who received GEMCAP chemotherapy. Twenty patients received Gem/Cap chemotherapy due to age or poor performance status, 14 (70%) completed the planned 3 months of chemotherapy with 4 having less than planned and 2 having additional cycles. No patients started with a dose reduction. Ten (50%) required a dose reduction during their chemotherapy course with 4 (20%) having some dose delay. Only 1 (5%) required discontinuation of chemotherapy. Significant toxicity was very modest with only 3 patients having any G3/4 toxicity. Only 1 (5%) patient had weight loss of greater than 10% of their body weight. The cause of death in the 1 patient was cancer progression and cachexia.
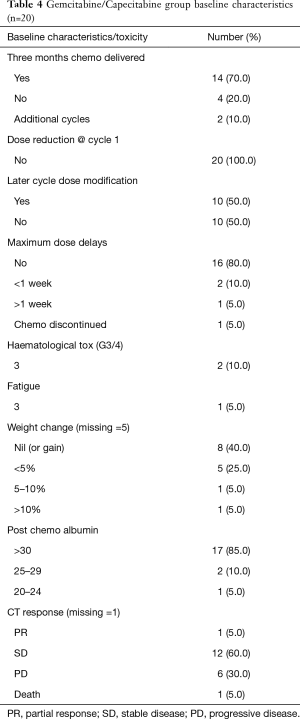
Full table
CRT group
In total 33 (38.8%) patients underwent CRT as part of their treatment protocol and details are outlined in Table 5 below. All patients completed the full course of radiotherapy. Two patients (6.1%) stopped the chemotherapy early due to toxicity (1 due to G3 weight loss and 1 due to G3 diarrhoea). Both of the patients who stopped chemotherapy were being treated with capecitabine.
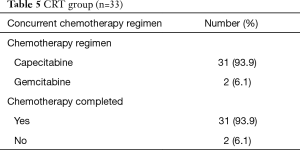
Full table
Efficacy
Resected group
Although locally advanced inoperable patients were included in this study (in order to record toxicity in the non-metastatic setting and calculate conversion rates) we were particularly interested in the tolerability and efficacy in the group of patients with resectable pancreatic cancer (please see consort diagram, Figure 2).
At the time the study started there were a small number of centres reporting efficacy in patients with borderline resectable tumours. The effect of FOLFIRINOX and chemo-radiotherapy on smaller, resectable tumours both in terms of response and influence on surgical morbidity was unknown (Figure 3).
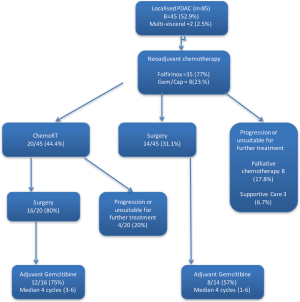
Outcome in the surgical sub group of patients
Forty five patients, following initial staging by CT CAP, MRI liver and EUS in the majority of cases, were considered to be potentially surgically resectable. Following induction chemotherapy and then chemo-radiotherapy if stable, 34 patients remained potentially operable. In 2 of cases there was previously unidentified evidence of metastatic disease found at operation and surgical bypass was carried out. In resected patient, pathological stage (Table 6) appeared to be most favourable in the group who had undergone chemotherapy, followed by CRT, however the result was not statistically significant and this was most likely because of the small sample size.

Full table
The same was seen for resection margin status (Table 7).

Full table
Overall survival
The median follow up for survivors was 21.2 (11.2–37.1) months. The median overall survival was 17.9 (13.2–22.6) months. The 12-month survival rate was 54% (SE 6%).
Median survival was 22.2 months in the potentially resectable group, very similar to the ESPAC 3 data previously discussed. It should be noted however that this group included those patients who did not proceed to surgery. Survival for patients proceeding to resection was 37 months.
The number of initially inoperable patients with C or D1 disease who could be down-staged to a resectable situation was only 3/19 (15.8%).
Location of primary tumour and weight loss during induction chemotherapy did not appear to impact on survival but pre-operative performance status and radiological tumour stage were significant determinants of survival on univariate analysis, with earlier tumours achieving the best results with neo-adjuvant treatment (Tables 8,9, Figure 4).
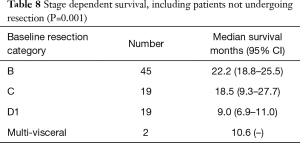
Full table
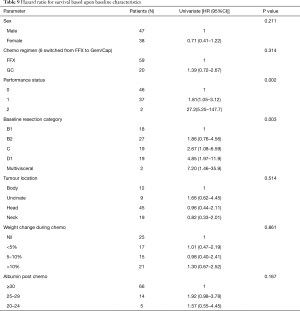
Full table
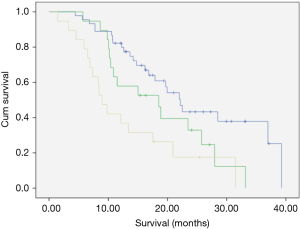
In the subgroup of patients undergoing surgical resection, survival was 37 months for patients initially thought resectable (B). For those with initially irresectable disease in whom down-staging was achieved and who proceeded to resection, survival was disappointing at 11.5 months (Table 10, Figure 5).

Full table
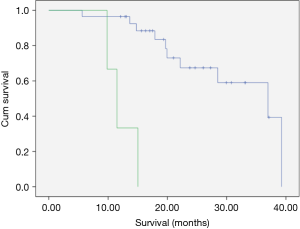
Survival for surgical patients
At time of data censoring April 29th 2016, 18/34 surgical patients were alive (56%). Median survival was 37.0 months (95% CI, 18.2–55.7).
We next examined whether the pre-operative treatment we delivered influenced the post-operative pathology specimen. We observed a statistically significant difference in LVI and less PNI and this fitted with the trend towards smaller sized tumour’s with a lower number of node positive tumour’s and also the reduced number of R1 cancers (previously described in Table 7).
Discussion
Our paper demonstrates the deliverability of a complex chemotherapy protocol in a cohort of patients with localized pancreatic cancer in the West of Scotland. It builds upon the current limited published data and also demonstrates that both FOLFIRINOX and chemo-radiotherapy can be delivered safely in the neo-adjuvant setting with acceptable levels of toxicity. The current ESPAC 5F study in the UK includes such regimes and it is of value to both the UK and wider clinical communities to see the impact of these regimes.
Only 19 patients (29.2%) stopped chemotherapy early in the FOLFIRINOX arm. Similarly 16 of 20 patients in the GEMCAP arm received all the planned cycles.
We were initially concerned about the impact of adjuvant gemcitabine chemotherapy in patients undergoing resection following neo-adjuvant chemotherapy and chemo-radiotherapy but found this to be well tolerated. The proportion of patients who received adjuvant chemotherapy was 66% overall. This is slightly higher than that reported in other adjuvant trials such as ESPAC 3 (2), or real world data such as the Scottish HBC cancer network audit.
One concern of a neo-adjuvant approach is the potential for patients to progress during this neoadjuvant phase and thus miss out on the possibility of surgery. However in our intention to treat cohort we demonstrate a median survival of 22 months, which is very similar to the median survival in the ESPAC 3 study. This indicates no overall detriment to patients treated by this algorithm. In addition it could be argued that avoiding futile surgery in those patients with very aggressive disease is in fact a benefit. Survival in the cohort of patients who progressed on neoadjuvant therapy was similar to that of patients not receiving adjuvant chemotherapy in the ESPAC III study (this data is not included in the paper).
Chemo-radiotherapy was well tolerated with all patients completing their radiotherapy and only two requiring their chemotherapy to stop early. This compare is very favourably with the recent SCALOP (11) study in which 29/72 patients had to stop radiotherapy early. This is likely to be a clear manifestation of advances in radiotherapy planning/delivery techniques as all of our patients group had their radiotherapy delivered with VMAT significantly reducing the radiation dose to regional organs at risk such as duodenum and liver and in addition our cohort included more earlier stage smaller tumours.
In the landmark ESPAC 3 (2) study the majority of patients had relatively high stage of disease with T3 N1 being by far the commonest pathological outcome. Only around 10% had stage I disease. In our resected cohort 8/34 (23.5%) had either T0N0 or T1N0 with 60% across the cohort having an R0 margin. It is hoped that with long-term follow we will demonstrate far better outcomes with such favourable pathology.
Our conversion rate of 15.8% is lower than that quoted in previous single centre experiences where rates of up to 30% have been reported (19). Via our classification a “B” tumour would be considered operable but with a high chance of positive margins or involved locoregional lymph nodes whereas a “C” was considered borderline resectable with a response to neoadjuvant treatment required for surgery to be considered. All of the patients classified as “C” had long segment SMV/portal vein involvement or significant (up to 180°) involvement of the SMA and were patients in whom surgical exploration would not have been attempted in our institution prior to the introduction of the neoadjuvant protocol. It is likely that some of the patients staged as “B” within our series would have been classed as borderline resectable in other series. The poor prognosis of patients initially staged as “C” in whom downstaging was achieved suggests that consideration should be given to accepting chemotherapy (± chemoradiotherapy) as definitive treatment regardless of response. This is certainly our approach to patients with proven metastatic disease at presentation. Clearly there are no randomized data to direct management of patients with “C” or D1’ tumours who respond to systemic therapy (11).
The number of patients treated remains small but in our experience at least, patients with initially inoperable disease are rarely downstaged and following surgical resection, survival was no better than for similar patients treated by chemoradiotherapy alone. Importantly the group that had the greatest survival was the B’s who managed the full treatment protocol. To our knowledge this is the most promising survival data for this cohort of patients and suggests that targeting earlier staged tumours i.e., potentially operable disease may offer some of the greatest benefits with the neo-adjuvant approach
Conclusions
In conclusion we have described a novel approach to localized pancreatic cancer patients in the west of Scotland. We have also demonstrated the potential benefits of chemotherapy and chemo-radiotherapy delivered in a neo-adjuvant setting including more favourable pathology, improved R0 rates and an indication of improved survival within the confines of acceptable toxicity. This work supports the aim of current neo-adjuvant studies such as ESPAC 5F but also goes further as it describes the combination of neo-adjuvant chemo + CRT not available within ESPAC 5F and extends experience to patients with initially resectable disease.
This paper has demonstrated encouraging survival in patients undergoing resection following neoadjuvant therapy. No study of adjuvant therapy has come close to demonstrating median survival of 37 months in pancreatic cancer. The PRIMUS 002 study, which was recently funded, and will be led by our centre will take these early clinical observations and attempt to correlate with a deeper pre- clinical understanding. In this novel neoadjuvant trial patients will have serial biopsies, with molecular profiling, and we will aim to identify the exceptional responders—but perhaps even more importantly the 30–50% who do not benefit from standard chemotherapy regimens and for whom alternative therapeutic options may become available in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As this was a service development rather than a clinical trial, formal ethical approval was not required and due to the retrospective nature of this study, the need for informed consent was waived.
References
- Cancer Statistics for the UK. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs. gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [Crossref] [PubMed]
- Jamieson NB, Chan NI, Foulis AK, et al. The prognostic influence of resection margin clearance following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Gastrointest Surg 2013;17:511-21. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Di Marco M, Di Cicilia R, Macchini M, et al. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? Oncol Rep 2010;23:1183-92. (Review). [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012;12:199. [Crossref] [PubMed]
- Mehta VK, Fisher G, Ford JA, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg 2001;5:27-35. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Ghaneh P, Wong WL, Titman A, et al. PET-PANC:multi-centre prospective diagnostic accuracy and clinical value study of PET/CT in the diagnosis and management of pancreatic cancer. Pancreatology 2016;16:S91. [Crossref]
- Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013;14:317-26. [Crossref] [PubMed]
- Millard T, Kunk PR, Ramsdale E, et al. Current debate in the oncologic management of rectal cancer. World J Gastrointest Oncol 2016;8:715-24. [Crossref] [PubMed]
- Vauthey JN, Dixon E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: rationale and overview of the conference. Ann Surg Oncol 2009;16:1725-6. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Schott AF, Hayes DF. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol 2012;30:1747-9. [Crossref] [PubMed]
- Jamieson NB, Denley SM, Logue J, et al. A prospective comparison of the prognostic value of tumor- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol 2011;18:2318-28. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Available online: https://www.acrin.org/portals/0/administration/regulatory/CTCAE_4.02_2009-09-15_quickreference_5x7.pdf
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Polistina F, Di Natale G, Bonciarelli G, et al. Neoadjuvant strategies for pancreatic cancer. World J Gastroenterol 2014;20:9374-83. [PubMed]