Neoadjuvant transarterial radiation lobectomy for colorectal hepatic metastases: a small cohort analysis on safety, efficacy, and radiopathologic correlation
Introduction
Colorectal cancer (CRC) is the fourth most prevalent malignancy in the United States with a 4.5% lifetime risk. Fifty percent of patients with CRC will develop metastatic liver disease (ml-CRC) which carries an untreated 5-year survival of 5–8% (1,2). Treatment options for ml-CRC include resection, systemic therapy, and loco-regional therapies. Although median overall survival for ml-CRC is approximately 30 months, some patients may achieve long-term disease control (3). Surgical resection is the gold standard treatment for ml-CRC with median 5-year survival rates after hepatectomy of 30–38% (4,5). Approximately 40% of patients experience recurrent hepatic disease after resection and up to 10% of patients recur within 6 months (6,7). Surgical candidacy requires a favorable performance status, liver dominant disease, adequate future liver remnant (FLR), and technically resectable lesions. Given these requirements, as many as 80% of patients with ml-CRC are not conventional surgical candidates (8).
Established neoadjuvant procedures designed to increase FLR include associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) and portal vein embolization (PVE). Both ALPPS and PVE generate FLR hypertrophy by redirecting portal vein flow from the diseased lobe and generating trophic factors that, inadvertently, may stimulate growth of the hepatic metastases (9-13). ALPPS involves a laparotomy with right portal vein ligation and parenchymal transection of the left lateral lobe. The patient is transferred to the ICU until sufficient FLR is obtained and a completion hepatectomy is performed. ALPPS generates prolific growth of FLR but carries perioperative morbidity and mortality rates as high as 68% and 9.6%, respectively (14,15). PVE involves the administration of embolic agents to the portal supply of the future resection site which typically generates FLR within 3–4 weeks. PVE has a technical success rate of 99%, morbidity of 2.5%, and mortality of 0.1% (16,17). PVE may have limited application in patients with ipsilateral portal vein thrombus (PVT) or clinically evident portal hypertension (9). There is controversy regarding the appropriate utilization of chemotherapy and PVE with studies suggesting tumor progression following suspension of systemic chemotherapy (17-20) and decreased FLR hypertrophy in patients on concurrent chemotherapy (21).
Radiation lobectomy (RL) with transarterial Yttrium-90 (Y-90) was first described by Siddiqi et al. in 2009 as a palliative treatment for ml-CRC (22). In distinction to conventional radioembolization, RL delivers intentionally ablative transarterial brachytherapy to both tumor and adjacent hepatic substrate. It is well-tolerated, generates FLR hypertrophy equivalent or greater to PVE, and can be used in patients with PVT or concurrent systemic chemotherapy (23,24). Contrary to PVE or ALPPS, RL has potential to control disease which permits FLR hypertrophy over several months allowing for surveillance of tumor biology. Early results demonstrate a histologic complete response in 33% of patients who received neoadjuvant RL prior to resection for primary and metastatic liver malignancy (25).
The purpose of this series is to report early single institution retrospective experience with RL for ml-CRC observing safety, efficacy, FLR hypertrophy, and radiopathologic correlation.
Methods
The study was performed as part of an existing IRB for hepatic loco-regional therapies. Four patients presenting with synchronous ml-CRC deemed inoperable due to inadequate FLR were treated with neoadjuvant systemic chemotherapy and RL after multidisciplinary tumor board consensus. Patients underwent mapping angiography, nuclear scintigraphy, and glass or resin Y-90 radioembolization based on treating physician preference. Patients underwent hepatectomy after a minimum of three months allowing for FLR hypertrophy and disease surveillance.
Eastern Cooperative Oncology Group (ECOG) functional status, Model for End Stage Liver Disease (MELD) scores, pre-procedure albumin-bilirubin (ALBI) grade, and Clavien-Dindo (C-D) complications related to RL and hepatectomy were analyzed. Contrast enhanced computed tomography (CECT) and magnetic resonance imaging (MRI) were utilized to evaluate RL efficacy according to Response Evaluation Criteria in Solid Tumors (RECIST) and modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria. Tumor response was categorized as stable disease (SD), partial response (PR), complete response (CR), and progressive disease (PD). Hepatic volumetric analysis was accomplished using Visage 7 (Pro Medicus Limited, California). Percentage FLR (%FLR) was calculated as left lobe volume (LLV)/total liver volume (TLV)] ×100. %FLR hypertrophy from baseline was calculated as [%FLR new - %FLR at baseline]/[%FLR at baseline] ×100. Pathologic analysis and radiopathologic correlation was performed retrospectively by a gastrointestinal pathologist and abdominal imaging radiologist with respective board certifications.
Case presentation
Case 1
A 51-year-old female presented with rectosigmoid cancer on screening colonoscopy and right hepatic lobe masses (10 and 6 cm). Neoadjuvant chemotherapy consisted of 5-fluorouracil, leucovorin and oxaliplatin (FOLFOX) with bevacizumab. A bevacizumab washout of at least four weeks was performed for all patients in this series. RL of the right hepatic lobe with glass Y-90 spheres and right lobar dose of 392 Gray (Gy) was performed two months after initiation of systemic therapy. Radioembolization was well tolerated and without clinical toxicity. She underwent an extended right hepatectomy three months after RL in addition to microwave ablation of a central left hepatic lobe lesion. There was a complete pathologic response in the right hepatic lobe specimen (Figures 1,2). The post-operative course was complicated by a bile leak at the microwave ablation site, which required percutaneous transhepatic biliary drainage (PTHD) and a Roux-en-Y hepaticojejunostomy. She ultimately developed progressive disease in the FLR 3 months after resection and died 14 months from diagnosis.
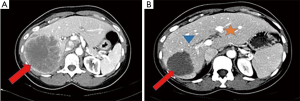
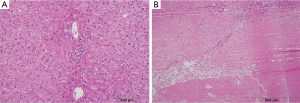
Case 2
A 30-year-old male presented with colonic polyposis, a large transverse colon tumor, and a solitary right hepatic lobe mass (5 cm). Neoadjuvant chemotherapy consisted of FOLFOX and bevacizumab. RL of the right hepatic lobe with glass Y-90 spheres and a right lobar dose of 235 Gy was performed three months after initiation of systemic therapy. Radioembolization was well tolerated and without clinical toxicity. Two and half months after the RL, the patient underwent laparoscopic total colectomy with ileorectal anastomosis; laparoscopic ultrasound-guided core liver biopsies of the treated lobe at the time of colectomy demonstrated no viable tumor. Six months after RL, the patient underwent extended right hepatectomy with a roux-en-y hepaticojejunostomy in addition to four microwave ablations and five wedge resections of the FLR for unexpected disease identified with intraoperative ultrasound. Histopathology demonstrated a complete response in the targeted mass but innumerable subcentimeter viable lesions that were occult to CECT. Postoperative course was complicated by recurrent bacteremia. He died from septic shock 11 months from diagnosis.
Case 3
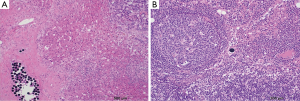
A 55-year-old male presented with rectal cancer, multiple right hepatic lobe metastases (measuring up to 3 cm), and atypical but biopsy-confirmed main right portal vein tumor thrombus. He received neoadjuvant chemotherapy with capecitabine and concurrent external beam radiotherapy of the rectal primary. RL of the right hepatic lobe with glass Y-90 spheres and right lobar dose of 206 Gy was performed three months after initiation of systemic therapy. The patient experienced mild fatigue, trace ascites, and slight increase in MELD score. Restaging MRI demonstrated necrotic tumor in the parenchyma and the portal vein (Figure 3). He received 4 cycles of FOLFOX and underwent a low anterior resection one and six weeks after RL, respectively. Uncomplicated right hepatectomy was performed nine months after RL given the anticipated systemic involvement in the setting of tumor thrombus. A complete histopathologic response was achieved in the liver and portal vein (Figure 4). The patient remains disease-free, off treatment, and at baseline performance 18 months from diagnosis.
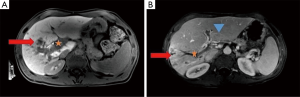
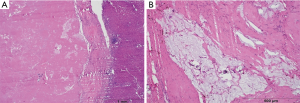
Case 4
A 63-year-old male presented with descending colon cancer and bilateral hepatic lobe masses (2.1–3.6 cm). He received neoadjuvant chemotherapy with FOLFOX and bevacizumab. Five months after diagnosis, he underwent laparoscopic left hemicolectomy and microwave ablation of a hepatic segment 3 metastasis. He underwent right hepatic lobe RL with resin Y-90 spheres and right lobar dose of 50 Gy, the maximum dose that could be administered due to vascular stasis, seven months after initiation of systemic therapy. RL was well-tolerated and without clinical toxicity. The patient underwent an extended right hepatectomy and segment 2 lesion ablation three months after RL. Histopathology demonstrated multiple lesions in the hepatectomy specimen with 20% necrosis (Figures 5,6). Post-operative course was complicated by intra-abdominal abscess requiring drainage, a right pleural effusion requiring pigtail placement, deep venous thrombosis, and failure to thrive. He died 13 months after diagnosis.
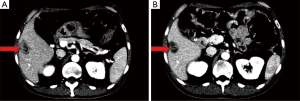
Results
Acute complications and biochemical response
There were only Grade 1 C-D complications after RL (Table 1). There was no significant change in ECOG at 1 or 3 months following RL (Table 2). Complete biochemical response was noted 3 months post-RL in all patients with elevated CEA (Table 3). All four patients tolerated RL well without significant hepatic toxicity. The median pre-RL MELD score was 6 at 1 month and 7 at 3 months. The pre-RL albumin-bilirubin (ALBI) score for patients 1–3 was A1 while patient 4 had an ALBI score of A2.
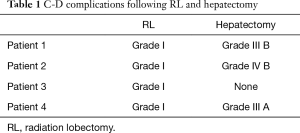
Full table
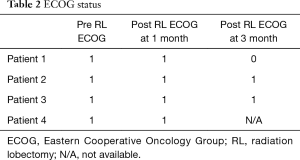
Full table
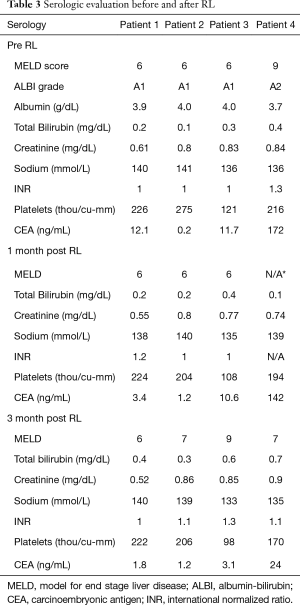
Full table
Volumetric response
The median %FLR hypertrophy between baseline and 3 months post-RL was 32.9% (range, 24.5–119%). The median %FLR at the time of surgery was 27% (range, 24–36.1%) (Table 4).

Full table
Radiologic and pathologic response
Enhancing and non-enhancing lesions were evaluated using mRECIST and RECIST, respectively (Table 5). Patient 1 and 3 had CR per mRECIST. Patient 4 had PR per mRECIST. Patient 2 demonstrated SD per RECIST.
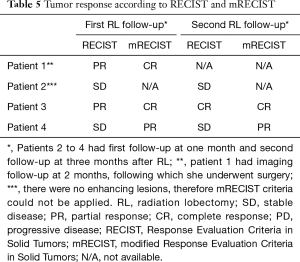
Full table
Pathologic findings including tumor response, Y-90 sphere distribution, and background liver parenchyma are discussed in Table 6. Patients 1 and 3 demonstrated a radiopathologic concordant complete response. Patient 2 had radiopathologic discordance with a complete response in the targeted lesion, but multiple imaging occult satellite lesions. Patient 4 approached radiopathologic concordance with 20% necrosis of lesions. There were differences in sphere distribution with glass spheres found singly in more peripheral intra and peritumoral vasculature while resin spheres aggregated in the central arterial and portal vasculature in large clusters (Figure 6).

Full table
Discussion
While only 20% of patients with ml-CRC are conventional surgical candidates, PVE and ALPPS have allowed for operative conversion in subjects with inadequate FLR. Neoadjuvant RL offers a unique advantage by delivering ablative transarterial brachytherapy in addition to generating FLR hypertrophy. The majority of patients in this series received RL doses greater than 200 Gy, higher than prior reported doses of 120-150 Gy in prior studies (23-25). Higher hepatic doses and concurrent systemic therapy were well-tolerated in these first line patients without significant clinical toxicity (grade I C-D complications) or hepatic dysfunction. Three out of four patients in the current study experienced grade III C-D complications after hepatectomy, similar to the 3 out of 4 patients with grade III C-D complications in another study (26). Three out of four patients received RL as an outpatient treatment. was equivalent or superior to FLR hypertrophy after RL was PVE but may be dependent on radiation dose (23,24). Patient 1 received the highest radiation dose and demonstrated the greatest %FLR hypertrophy, while patient 4 received the lowest dose and demonstrated the lowest hypertrophy. Patient 3 demonstrated a slow trajectory of FLR hypertrophy over 9 months but was ultimately adequate to support surgical resection. This may be due to a preexisting compensation for the reduced hepatic function in the affected lobe.
In addition to FLR hypertrophy, ablative transarterial brachytherapy is tumoricidal and provides local disease control. 100% local disease control was achieved in our experience with 50% of patients demonstrating complete pathologic response after RL and systemic chemotherapy. Patient 3 had the most advanced presentation of ml-CRC with atypical PVT but demonstrated the best overall outcome with a complete pathologic response and longest survival which supports the notion of biologic test of time in patients with ml-CRC. Two out of four patients progressed after resection at 3 months and may have benefited from longer surveillance. Unlike PVE, patients in this study had a minimal time to resection of 3 months (25). This increased observational period may circumvent an unnecessary resection should there be FLR disease progression that cannot be addressed (7,26). Patient 1 would have avoided surgical complications if the team had 6 months data which would have demonstrated FLR disease and potentially obviated surgery. Survival outcomes in our small series were impacted by management of metastatic disease in the FLR, which is why understanding tumor biology remains paramount. The ideal post-RL surveillance prior to resection needs further investigation and there may be benefit from optimizing control of the primary tumor to avoid continual metastatic deposition, given the histopathologic findings of patient 2.
Histopathology demonstrated hepatic parenchymal hemorrhage in the immediate peritumoral liver with associated hepatocyte dropout, hemosiderin-laden macrophages but an unexpected paucity of fibrosis in the background liver. The lack of hepatic fibrosis may explain the lack of hepatic dysfunction or manifestations of portal hypertension after RL in our first line cohort who otherwise had normal liver substrate. Glass Y-90 spheres were seen singly, distributed widely within the target lesion. Resin Y-90 spheres aggregated in large clusters within the proximal vasculature supplying the target lesion and in portal vessels away from tumor. Resin spheres were also found within hilar lymph nodes but without an associated tissue reaction, which suggests absent radioactivity at the time of exposure. A comparison between glass Y-90 and resin Y-90 may be needed to determine the impact of sphere distribution and specific activity on tumor response. There was radiopathologic concordance in two out of four patients; patient 4 approached radiopathologic concordance, while patient 2 demonstrated radiopathologic discordance.
Challenges to RL include operating in an irradiated field, which may present with increased inflammation adjacent to the treated parenchyma. However, this challenge has been addressed with minor changes in surgical technique (25).
Conclusions
Neoadjuvant RL was well tolerated and provides promising disease control with ample FLR hypertrophy. Complications were mainly due to intraoperative management of metastatic disease in the FLR. RL may have an advantage over PVE and ALPPS in the setting of vascular invasion and uncertain disease biology. Surgical patient selection of ml-CRC after RL requires further study. A 50% CR raises the possibility of definitive chemoradiation in poor surgical candidates.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Toskich and Dr. Geller are consultants for BTG.
Informed Consent: This study is part of an existing IRB at Shands Hospital at University of Florida and since these patients were retrospectively evaluated, the IRB provided us an exemption for requiring consent.
References
- SEER Cancer Statistics Review (CSR) 1975-2013. Available online: https://seer.cancer.gov/csr/1975_2013/
- Treatment of Colorectal Cancer. 2010. Available online: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hematology-oncology/treatment-of-colorectal-cancer/
- Venook AP, Niedzwiecki D, Lenz HJ, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 32:5s, 2014 (suppl; abstr LBA3).
- Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 2006;94:982-99. [Crossref] [PubMed]
- Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 2012;4:283-301. [PubMed]
- Battula N, Tsapralis D, Mayer D, et al. Repeat liver resection for recurrent colorectal metastases: a single-centre, 13-year experience. HPB (Oxford) 2014;16:157-63. [Crossref] [PubMed]
- Viganò L, Capussotti L, Lapointe R, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol 2014;21:1276-86. [Crossref] [PubMed]
- Penna C, Nordlinger B. Surgery of liver metastases from colorectal cancer: new promises. Br Med Bull 2002;64:127-40. [Crossref] [PubMed]
- May BJ, Madoff DC. Portal vein embolization: rationale, technique, and current application. Semin Intervent Radiol 2012;29:81-9. [Crossref] [PubMed]
- Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 2004;5:836-47. [Crossref] [PubMed]
- Schweizer W, Duda P, Tanner S, et al. Experimental atrophy/hypertrophy complex (AHC) of the liver: portal vein, but not bile duct obstruction, is the main driving force for the development of AHC in the rat. J Hepatol 1995;23:71-8. [Crossref] [PubMed]
- Seymour K, Charnley RM, Rose JD, et al. Preoperative portal vein embolisation for primary and metastatic liver tumours: volume effects, efficacy, complications and short-term outcome. HPB (Oxford) 2002;4:21-8. [Crossref] [PubMed]
- Elias D, De Baere T, Roche A, et al. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg 1999;86:784-8. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Hasselgren K, Sandström P, Björnsson B. Role of associating liver partition and portal vein ligation for staged hepatectomy in colorectal liver metastases: a review. World J Gastroenterol 2015;21:4491-8. [PubMed]
- Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg 1986;10:803-8. [Crossref] [PubMed]
- van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. [Crossref] [PubMed]
- Hoekstra LT, van Lienden KP, Doets A, et al. Tumor progression after preoperative portal vein embolization. Ann Surg 2012;256:812-7; discussion 817-8. [Crossref] [PubMed]
- Simoneau E, Aljiffry M, Salman A, et al. Portal vein embolization stimulates tumour growth in patients with colorectal cancer liver metastases. HPB (Oxford) 2012;14:461-8. [Crossref] [PubMed]
- Pamecha V, Levene A, Grillo F, et al. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer 2009;100:617-22. [Crossref] [PubMed]
- Beal IK, Anthony S, Papadopoulou A, et al. Portal vein embolisation prior to hepatic resection for colorectal liver metastases and the effects of periprocedure chemotherapy. Br J Radiol 2006;79:473-8. [Crossref] [PubMed]
- Siddiqi NH, Devlin PM. Radiation lobectomy-a minimally invasive treatment model for liver cancer: case report. J Vasc Interv Radiol 2009;20:664-9. [Crossref] [PubMed]
- Vouche M, Lewandowski RJ, Atassi R, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol 2013;59:1029-36. [Crossref] [PubMed]
- Teo JY, Allen JC Jr, Ng DC, et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB (Oxford) 2016;18:7-12. [Crossref] [PubMed]
- Lewandowski RJ, Donahue L, Chokechanachaisakul A, et al. (90) Y radiation lobectomy: Outcomes following surgical resection in patients with hepatic tumors and small future liver remnant volumes. J Surg Oncol 2016;114:99-105. [Crossref] [PubMed]
- Henry LR, Hostetter RB, Ressler B, et al. Liver resection for metastatic disease after y90 radioembolization: a case series with long-term follow-up. Ann Surg Oncol 2015;22:467-74. [Crossref] [PubMed]


