Treatment of advanced Gall bladder cancer in the real world—can continuation chemotherapy improve outcomes?
Introduction
India has one the highest incidences of Gallbladder Cancer (GBC) in the world, as per registry and GLOBOCAN data, with approximately 18,727 new cases in India per year (1). Based on three well conducted randomized controlled trials, the current standard of care for advanced GBC (A-GBC) is a Gemcitabine-Platinum (G-P) doublet which entails a median survival of 9.5–11.7 months (2,3). However, there has been minimal progress beyond this point in terms of survival as newer combinations and targeted therapies have not shown benefit above the current standard palliative chemotherapy (4).
It remains imperative to improve upon current first line treatment strategies in these patients as they will invariably progress within a short span of time upon cessation of chemotherapy. This is particularly important as only 15–35% of patients progressing on 1st line chemotherapy receive second line chemotherapy (CT2), which itself has not been standardized yet (5,6). Data previously published from our institution shows that only 19.5% of patients receiving first line palliative chemotherapy, receive second line treatment (7).
One of the strategies that has gained ground, especially in lung cancer, is the concept of maintenance or continuation of chemotherapy after 4–6 cycles and this has shown an overall survival (OS) benefit in the large PARAMOUNT study (8). While there is no such evidence in GBC, it does entail an evaluation of continuing treatment in patients responding to first line chemotherapy with fair tolerance, especially considering the lack of effective second line options.
At our institution, we treat approximately 180 A-GBC patients per year, including about 160 patients with advanced, metastatic disease. We continue first line palliative chemotherapy in patients who have achieved at least stable disease after 6–8 cycles of chemotherapy as per institutional policy. We evaluated whether this is a feasible, viable option in A-GBC. We also attempted to derive a clinical tool based on variables used in the clinic to predict median survival.
Methods
Database and patient population
Data for this study was extracted from a prospectively maintained GBC database. The study for evaluation of metastatic and locally A-GBC treated with palliative chemotherapy was approved by the Institutional Review Board (IRB) and Ethics Committee (EC) (IEC-0216/1613/001).
Consecutive series of patients diagnosed with A-GBC, between Jan 2013 to June 2015 were evaluated for first line chemotherapy after being discussed in the Multidisciplinary Joint Clinic (MDJC). After evaluation for fitness for chemotherapy, they were treated with first line palliative chemotherapy, either Gemcitabine-Cisplatin (GC) or Gemcitabine-Oxaliplatin (GO) (with standard schedules as previously published) (3,9). Demographic and clinical details of the entire cohort were recorded and analysed for event free survival (EFS) and OS. Patients who achieved and maintained CBR, at the end of 6–8 cycles, were then evaluated for continuation of chemotherapy with ongoing supportive care or further observation with supportive care only.
The decision for continuing chemotherapy was based on an extensive discussion between treating physician and patients with regard to further goals and management plan. Tolerance to previous chemotherapy, adverse events and the need for admission during prior chemotherapy, was taken into consideration when explaining treatment strategy to patients. The decision to continue chemotherapy was solely based on patient’s choice and decision for either option.
Dose modifications and withholding of chemotherapy were subject to treating physician, based on established guidelines.
Outcome variables
Response assessment on treatment was carried out every 2–3 months by clinical evaluation and CT scans and reported using RECIST criteria, version 1.1, where feasible (10). When application of RECIST was not feasible, response was not quantified. Response rates (RR) and clinical benefit rate (CBR) were reported as percentages. EFS was calculated as the time between start of therapy and the date of progression, loss to follow up, cessation of chemotherapy due to Grade 3/4 adverse events or death from any cause (in case the disease had not progressed). OS was calculated as the time between start of therapy and the date of death due to any cause or loss to follow up.
Statistical analysis
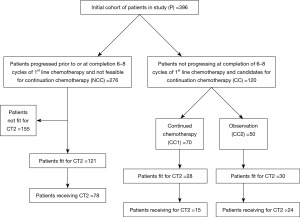
Descriptive statistics including median, frequency and percentage for categorical variables is used to describe age, gender distribution, treatment, response to treatment and toxicities. Median EFS and median OS were estimated by Kaplan-Meier methods. For the purposes of comparing outcomes, the entire cohort (P) was divided into three overlapping mutually non-exclusive groups as explained in consort diagram (Figure 1).
Hazard Ratios (HR) for survival and two-sided 95% CI were computed with unadjusted and adjusted Cox proportional hazards model for group comparisons for the entire cohort. Prognostic factors for EFS for the entire cohort were evaluated by log rank test and included age (≤50 vs. >50 years), gender (female vs. male), surgery (prior history of curative surgery vs. no surgery), obstructive jaundice (absence vs. presence), albumin (<3.5 vs. ≥3.5 gm/dL), Eastern Cooperative Oncology Group (ECOG) (0–1 vs. ≥2), disease burden (one site vs. >1 site of metastases), leucocytosis (presence vs. absence; Upper limit of Normal −10×109/L) and liver metastases (presence vs. absence). The above mentioned factors were also evaluated as prognostic factors for OS, with the addition of evaluating the benefit of CT2 [(those eligible vs. those who were not eligible) and (those able to receive 2nd line chemotherapy vs. those did not receive the same)].
Eligibility for CT2, as opposed to the actual number of patients receiving CT2 was evaluated as a factor for OS. Patients may not have opted for CT2 despite being fit for the same as CT2 is not currently standardized, the benefits are suspect and survival advantage over supportive care only has not been proven.
Multivariate analysis was performed for all variables for EFS and OS when factors assessed for univariate analysis approached or achieved statistical significance (P value approaching 0.2).
Comparison for EFS and OS was done between CC1 and CC2 with two sided log-rank test. The role of continuation chemotherapy was added to multivariate analysis when performed for the entire cohort as well.
Results
Treatment groups, RR and survival
In the specified period, 396 patients were diagnosed with A-GBC were planned for palliative chemotherapy at our MDJC. All patients who received at least one cycle of chemotherapy in TMH were included for analysis.
Entire population (P)
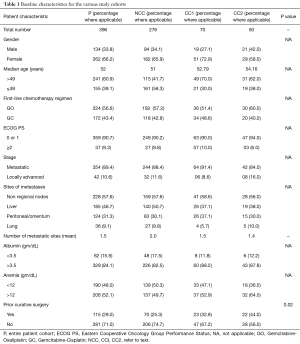
Baseline clinical characteristics of this cohort as well as patient groups are detailed in Table 1.
Full table
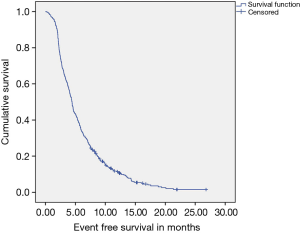
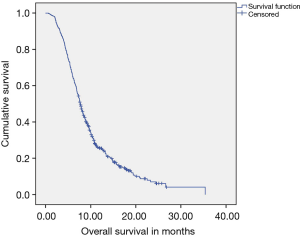
A total of 224 patients (56.6%) received GO as first line therapy, while 172 patients (44.4%) received GC. A median of five cycles of therapy was delivered to patients as first line prior to event as defined. With a median follow up of 17.2 months, Median EFS (Figure 2) of the cohort was 4.53 months (95% CI: 4.23–4.83), while median OS (Figure 3) was 7.65 months (95% CI: 7.14–8.16).
N-CC patient population
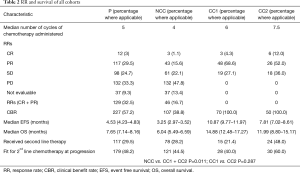
A total of 276 patients (n=396; 69.6%) did not achieve or maintain at least a stable disease on response evaluation at the completion of 6–8 cycles of first line chemotherapy (Table 2).
Full table
Patients were able to receive a median of four cycles of chemotherapy. RR was 16.7% and CBR of 38.8%. The median EFS of the cohort (Figure S1) was 3.25 months (95% CI: 2.97–3.52), while median OS (Figure S2) was 6.04 months (95% CI: 5.49–6.59).
CC patient population (Table 2)
A total of 120 patients (n=396; 30.3%) were able to maintain at least SD after 6–8 cycles of 1st line palliative chemotherapy. These patients were candidates for continuation chemotherapy (CC cohort). The outcomes of patients in this group are reported as two separate cohorts (as previously described).
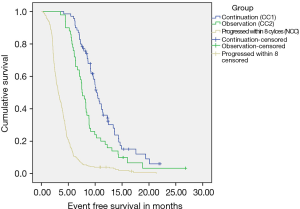
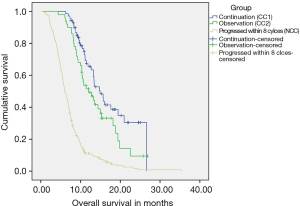
(I) CC-1: 70 patients (n=396; 17.67%) continued to receive chemotherapy after 6–8 cycles till occurrence of an event. These patients received a median of 6 cycles of chemotherapy before receiving continuation chemotherapy. RR was 72.9% and CBR of 100%. With a median follow up of 17.9 months, Median EFS of the cohort was 10.87 months (95% CI: 9.77–11.97), while median OS was 14.88 months (95% CI: 12.48–17.27). These patients were able to receive a median of 4 cycles of continuation chemotherapy (range, 2–15) (Figures S1,S2). The details of the continuation chemotherapeutic regimens received are as per Table S1.

Full table
(II) CC-2: 50 patients (n=396; 12.6%) did not receive chemotherapy after 6–8 cycles and were further observed till evaluation of treatment at progression. These patients received a median of 7.5 cycles of chemotherapy before continuing on observation. The RR was 64% and CBR was 100%. With a median follow up of 14.9 months, Median EFS of the cohort was 7.81 months (95% CI: 7.02–8.61), while median OS was 11.99 months (95% CI: 8.80–15.77).
Details of CT2
The feasibility of receiving CT2 in CC1 vs. CC2 was not affected whether patients received continuation chemotherapy after 6–8 cycles or were observed (P=0.287). The details are as per Table 2.
Predictive and prognostic factors
Factors for EFS (Table S2)
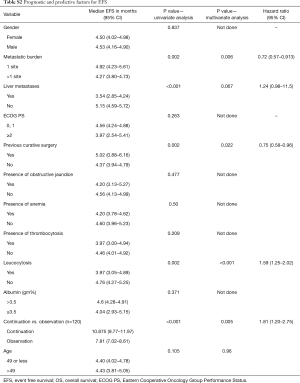
Full table
On univariate analysis of prognostic factors for EFS, presence of single site of metastases (P=0.002), absence of liver metastases (P<0.001), prior curative surgery (P=0.002), absence of leucocytosis (P=0.002) and the use of continuation chemotherapy (P<0.001) attained statistical significance for predicting improved EFS. On multivariate analysis, single site of metastases [P=0.002; HR 1.521 (95% CI: 1.160–1.994)], prior curative surgery [P=0.022; HR 0.75 (0.58–0.96)] absence of leucocytosis [P<0.001; HR 2.22 (1.672–2.958)], and administration of continuation chemotherapy [P<0.001; HR 4.003 (3.166–5.062)] maintained their statistical significance in predicting superior EFS.
Factors for OS (Table S3)
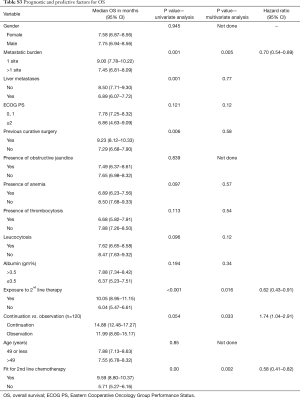
Full table
On univariate analysis of prognostic factors for OS, presence of single site of metastases (P=0.001), absence of liver metastases (P=0.001), absence of leucocytosis (P=0.096) prior curative surgery (P=0.022), use of continuation chemotherapy (P=0.05), fitness for CT2 (P=0.00) and exposure to CT2 (P<0.001) attained statistical significance for predicting improved OS. On multivariate analysis, single site of metastases [P=0.005; HR 0.70 (0.54–0.89)], administration of continuation chemotherapy [P<0.001; HR 3.672 (2.654–5.079)], fitness for CT2 [(P=0.002; HR 0.58 (0.41–0.82)] and exposure to CT2 [P<0.001; HR 2.657 (2.139–3.299)] maintained their statistical significance in predicting superior OS.
Clinical tool for predicting outcomes (Figure S3)
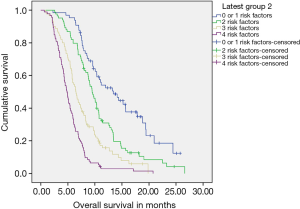
Four statistically significant factors affecting OS were drawn from our multivariate analysis—single site of metastases, administration of continuation chemotherapy, being fit for CT2, exposure to CT2 (Table S3). The absence of 0–1, 2, 3 or all 4 factors predicted for a median OS of 13.04, 9.49, 6.47 and 4.86 months respectively.
Discussion
A-GBC has a high incidence in particular geographical areas, with certain regions in India (New Delhi, Kamrup etc.) being amongst them (11). The Indian Council of Medical Research (ICMR) consensus document for the management of GBC was an initial step in forming region specific guidelines, but there continues to be an unmet need in terms of cost effective as well as investigational strategies to improve outcomes in this group of cancers (12). To the best of our knowledge, this study represents the largest single centre experience of A-GBC treated with first line palliative chemotherapy.
A total of 396 patients were treated over 2.5 years by the Gastrointestinal and Hepatobiliary Cancer Disease Management Group (DMG) at a high volume tertiary cancer centre. For the purposes of comparison and perspective, we have shown the baseline characteristics and outcomes of the four doublet cohorts from the seminal studies which were instrumental in establishing GC (ABC-02 and BT22) and GO (Andre et al. and Sharma et al.) as standard first line palliative chemotherapy (2,3,13) (Table S4). Our cohort was approximately a decade younger compared to the European cohorts, while being similar to the population from the study by Sharma et al. (52 vs. 49 years). Another notable difference was the lower percentage of non-metastatic patients in our study (10.6%), compared to the two studies where this data was available (ABC-02—27%; Andre et al.—27.1%), although the number of patients undergoing prior curative surgery varied widely across the studies (18.1–54.3%).
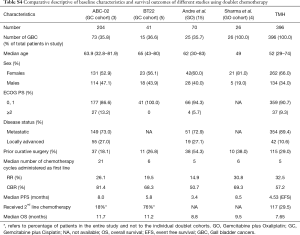
Full table
Since it is a retrospective cohort, the first line chemotherapy option was as per physician choice, with patients receiving GO than GC (220 vs. 176 patients, P= NA). We were able to administer a median of 5 cycles of chemotherapy before occurrence of an event and this was similar to that administered across the trials. Patients in our cohort showed an overall CBR comparable to those shown with GO in the study by Andre et al. (50.7% vs. 57%) and better than the GBC cohort in the same study (43.4%). There is no direct comparison between GC and GO as first line therapy; the outcomes in this study may reflect the predominantly GO treated nature of the study cohort (12,13). The median EFS and OS in this study were 4.53 and 7.65 months, respectively. While this is lower than the OS shown in trials and may be explained by differences in trial and real world populations, it would be prudent to remember that the meta-analysis of 104 studies by Eckel et al., albeit almost a decade back, showed a median OS of 9.3 months with doublet chemotherapy and only 7.2 months for GBC cohort (14). Additionally, the higher number of purely metastatic patients in our study (89.4%) as previously mentioned and the intrinsically inferior survival in GBC with palliative chemotherapy compared to other subsets of BTC (Andre et al.—6.1 vs. 11.0 months, Sharma et al.—9.5 months, BT 22—9.1 vs. 13 months) may partially explain these outcomes.
An interesting hypothesis generating concept that emerged from this study is that of continuing chemotherapy in patients who maintain response at the completion of 6–8 cycles of chemotherapy. This practice in our institution is based on recognizing the rapid deterioration of GBC patients on progression, thereby missing the opportunity for further treatment as well as the recognition of lack of a standardized second line option. A total of 120 patients (30.3%) appeared to be candidates for continuing chemotherapy, of which 70 patients (n=120; 17.67%) actually continued chemotherapy, while the remaining (n=120; 12.6%) were observed. Surprisingly, despite no obvious intent to match these patients, both groups appeared largely similar for baseline characteristics, except for the percentage of patients undergoing curative surgery in the observation cohort. Within the confines of a retrospective analysis, there was a statistically significant difference in median OS between these two groups (continuation—14.88 vs. 11.99 moths; P=0.033). While it can argued that we are comparing outcomes in a biologically selected group of patients, it also ensures maximizing outcomes for this group of responding patients. A statistically significant difference in OS was maintained between these two groups with an equal percentage of patients in both groups being fit for CT2. However, besides the obvious stated reason as to informed patient choice of continuing chemotherapy, we are unable to offer any other clinical or biological reason for this natural separation into these two cohorts. A possible clue might lie in the actual tolerance and adverse events profile of these patients while receiving first line chemotherapy, thereby influencing their decision against or for further chemotherapy. We do not have comprehensive information on the same, but suggest an evaluation of this approach in a clinical trial. Our analysis of prognostic factors allowed us to formulate a practical model that can be used easily in clinical practice. The factors we analysed have been previously examined as potential prognostic factors, either in BTC or advanced cancers of different tumors. Leucocytosis has been examined individually and has predicted for shorter survival in terminally ill patients with advanced cancers. Whether leucocytosis is a marker of necrosis and inflammation, metastatic spread, or a stress response to the tumor are assumptions that have not been completely explained (15).
Metastatic burden is a biologically feasible indicator of disease status and it is not unexpected that those patients with a single site of disease did better than those with multiple sites of disease. Importantly, fitness for and receipt of CT2 appear to be separately important factors in the clinic. However, patient’s choice, is an important overlooked component of further therapy, especially when the therapy is associated with suspect benefits, as in the case of GBC. A reasonably fit patient might decline CT2 as an informed decision, when explained the magnitude of risks and benefits of the same. While he will not receive the potential advantage of CT2, the role of initial first line and continuation chemotherapy in improving or maintaining his fitness even after progression, is suggested by this variable. Such clinical indicators should be kept in mind while treating patients in the non-trial setting. The factors identified in our analysis, while strictly not prognostic or predictive, allow the clinician to make expected survival assessments at multiple steps in the management course of a patient with GBC
We do acknowledge important caveats our study. Loss to follow up rate was 9.3%, predominantly in the subset which progressed prior to completion of 6–8 cycles of therapy (no loss to follow up in CC1 + CC2 cohort). The heterogeneity in first line palliative chemotherapy as well as continuation chemotherapy is a confounding factor that requires a randomized trial for verification. Besides patient’s choice on continuing chemotherapy versus observation, further explanation for this is not available in this study. The lack of documentation of adverse events hampers any assessment of tolerance, thereby limiting our ability to explain outcomes to only that by survival. Tolerance and quality of life are important considerations in palliative chemotherapy and our analysis does not comment on this aspect.
To conclude, our study of 396 patients with A-GBC is an attempt to show outcomes with palliative chemotherapy in the real world setting. The median OS is lower than published evidence, but we are able to identify a cohort of patients whose outcomes can be maximized by continuation of chemotherapy. We suggest a multi-step assessment of patient variables by the clinician in predicting expected patient outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study for evaluation of metastatic and locally A-GBC treated with palliative chemotherapy was approved by the Institutional Review Board (IRB) and Ethics Committee (EC) (IEC-0216/1613/001).
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014;25:391-8. [Crossref] [PubMed]
- Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010;28:4581-6. [Crossref] [PubMed]
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [Crossref] [PubMed]
- Walter T, Horgan AM, McNamara M, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer 2013;49:329-35. [Crossref] [PubMed]
- Lamarca A, Hubner RA, David Ryder W, et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328-38. [Crossref] [PubMed]
- Ramaswamy A, Ostwal V, Pande N, et al. Second-Line Palliative Chemotherapy in Advanced Gall Bladder Cancer, CAP-IRI: Safe and Effective Option. J Gastrointest Cancer 2016;47:305-12. [Crossref] [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Murthy NS, Rajaram D, Gautham MS, et al. Trends in incidence of gallbladder cancer—Indian scenario. Gastrointestinal Cancer: Targets and Therapy 2011;1:1-9.
- Shukla HS, Sirohi B, Behari A, et al. Indian Council of Medical Research consensus document for the management of gall bladder cancer. Indian J Med Paediatr Oncol 2015;36:79-84. [Crossref] [PubMed]
- André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339-43. [Crossref] [PubMed]
- Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896-902. [Crossref] [PubMed]
- Connolly GC, Khorana AA, Kuderer NM, et al. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res 2010;126:113-8. [Crossref] [PubMed]