Updated survival outcomes and analysis of long-term survivors from the MORE study on safety and efficacy of radioembolization in patients with unresectable colorectal cancer liver metastases
Introduction
Colorectal cancer is the third most commonly diagnosed cancer in the United States (U.S.) for both men and women, and the second most common cause of cancer-related mortality. It is estimated that in 2016, 134,490 new cases of colorectal cancers will be diagnosed in the U.S. and 49,190 people will die from this disease (1). The 5-year survival rate for patients diagnosed with localized disease is 90%; however, the 5-year survival rate for patients diagnosed with metastatic disease is only 13% (1). The most common site of metastasis is the liver (2). More than half of patients diagnosed with colorectal cancer present with or eventually develop liver metastases, only 10%–20% of which are resectable at presentation. A percentage of these metastases may be downstaged for surgery by treatment with chemotherapy and targeted agents (2,3). However, patients with liver metastases which remain unresectable have a poor prognosis and are generally given second-line chemotherapy, with a median survival of approximately one year (3).
For patients with unresectable colorectal cancer liver metastases that are refractory to first-line chemotherapy, a variety of interventional radiological procedures may also be considered (4-6). One such treatment is radioembolization (RE), also known as selective internal radiation therapy (SIRT), with yttrium-90-labeled (90Y) microspheres. This treatment modality consists of the selective delivery of radioactively labeled particles to liver tumors via the hepatic artery. Selective delivery of 90Y microspheres to tumors can be achieved because hepatic tumors receive most of their blood supply from the hepatic artery, while normal hepatic tissue receives blood mainly from the portal vein (4,7).
In 2015 first results from the Metastatic colorectal cancer liver metastases Outcomes after RadioEmbolization (MORE) study, a retrospective analysis of 606 patients with unresectable colorectal liver metastases treated with RE using 90Y-labeled resin microspheres (SIR-Spheres®) were published. Patients were treated between July 2002 and December 2011 at one of 11 tertiary care centers in the U.S., and received a median of 2 (range, 0-6) lines of chemotherapy prior to treatment with RE. At a median follow-up of 8.6 months, median survivals of patients following RE as a 2nd-line, 3rd-line, or 4th-plus line therapy were 13.0 (range, 10.5–14.6), 9.0 (range, 7.8–11.0), and 8.1 (range, 6.4–9.3) months, respectively (8). These survival times compare favorably to those of patients treated with chemotherapy and other systemic therapies in similar settings (8-11). This initial analysis concluded that 90Y-RE appeared to have a favorable survival benefit for these patients. Importantly, it noted an apparent favorable survival benefit even for patients who had received ≥3 lines of prior chemotherapy (90Y-RE as a 4th-plus line treatment) (8). Subsequent analyses from the MORE study have examined the safety and efficacy of 90Y-RE within the subgroup of elderly patients ≥70 years, and have examined baseline patient characteristics associated with long-term survival. A summary of key points from previous publications from the MORE study is given in Box 1.

Full table
This paper reports updated survival outcomes from the MORE study through September 15, 2016, reflecting an additional 138 person-years of follow-up from the first published analysis. A sub-cohort of long-term survivors who were still alive a year or more after first RE treatment are identified, and sub-analysis of these long-term survivors with a focus on baseline patient characteristics and treatment-related factors associated with long-term survival is reported as well. Our overall analysis contributes to a developing body of literature from the original MORE study, which continues to be one of the largest studies to date of metastatic colorectal cancer patients who received 90Y-RE alone after failed lines of chemotherapy.
Methods
Study design, patient selection, and treatment
The MORE study (clinicaltrials.gov identifier: NCT01815879) was an investigator-initiated retrospective study of 606 patients with colorectal cancer liver metastases who were consecutively treated with RE using 90Y resin microspheres (SIR-Spheres®). Patients were treated between July 2002 and December 2011 at one of 11 U.S. tertiary care centers, and data at each site were collected from source documentation by an independent contract research organization. Each site was granted institutional review board exemptions prior to data collection. All patients with a diagnosis of metastatic colorectal cancer who had received at least 1 RE treatment and 1 follow-up visit were included in the analysis.
Centers were guided in the selection of patients, pre-treatment work-up, and RE by the published consensus from the Radioembolization Brachytherapy Oncology Consortium (REBOC) and earlier reviews (15-17). In summary, 90Y-RE was considered for advanced liver-only or liver-dominant metastatic colorectal cancer, which was deemed not suitable for surgery, ablation, or systemic therapy, and which had progressed or become refractory to at least one line of systemic therapy. Candidates for 90Y-RE had Eastern Cooperative Oncology Group (ECOG) performance status score of up to 2 and untreated life expectancy of at least 12 weeks. Patients with signs of liver failure or compromised bone marrow or pulmonary function were considered unsuitable for 90Y-RE. However, under exceptional circumstances and with informed consent, some patients were treated outside the outlined criteria based on the clinical judgement of individual treating physicians.
Data collection and analysis
Patient medical charts and/or public records were accessed to obtain date of death (DOD). Adverse events (AEs), baseline patient tumor characteristics, and treatment histories were previously collected as described (8,14). Briefly, data were collected at baseline, on the day of the first 90Y-RE treatment (day 0), and at all subsequent visits or until death. All results from liver function, hematologic, and blood biochemistry tests were recorded and submitted to a centralized data collection center. The National Cancer Institute Common Toxicity Criteria Adverse Events version 3.0 was used as a tool to grade both the nature and severity of all AEs (18). Survival was calculated with the first day of 90Y-RE treatment serving as day 0 to the day of death or last follow-up.
Statistical analysis
Overall and stratified survival were estimated by the Kaplan-Meier method, and the log-rank test used to assess statistical significance. Median survival (months) and the 95% confidence interval were reported. Comparisons of prognostic variables and survival at 1 year (yes, no) include ANOVA for continuous variables, Fisher’s exact test for dichotomous variables, Chi-square general association for categorical variables, and Wilcoxon rank sum test for ordinal variables. P-values for this analysis exclude patients who did not survive 1 year and were censored, and consistency of survival comparisons in all patients was confirmed.
P-values were calculated as follows: for continuous variables, ANOVA; for dichotomous variables, Fisher’s exact test; for nominal categorical variables, Chi-Square general association test; for ordinal variables, Wilcoxon rank sum test.
Results
Updated survival analysis of the entire MORE cohort
In this paper, we report extended survival surveillance of the remaining MORE patients through September 15, 2016, at a median follow-up of 9.5 months. During this analysis, death dates were obtained for an additional 71 patients. In all, death dates were confirmed for 574 patients out of 606, or 95% of all enrolled patients. Updated Kaplan-Meier estimates of OS were reported at half-year intervals through 5 years (Table 1). The survival percentage at 1, 2, and 3 years following 90Y-RE was 45%, 18.9%, and 7.0%, respectively. The updated overall median survival was 10.0 months (95% CI: 9.2–11.8 months) at a median follow-up of 9.5 months versus the originally reported 9.6 months (95% CI: 9.0–11.1 months) at a median follow-up of 8.6 months as reported in the first MORE study analysis (8).
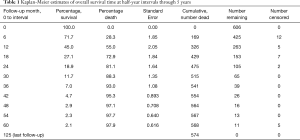
Full table
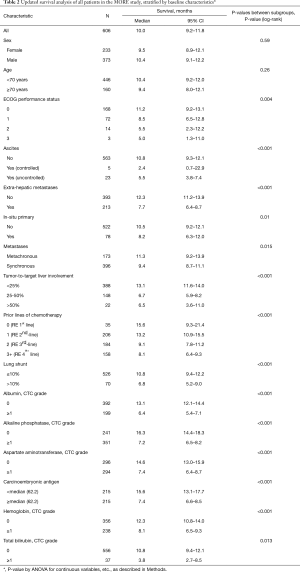
An updated survival analysis of patients stratified by baseline patient characteristics and treatment factors was also performed (Figures 1,2 and Table 2). Factors significantly associated with patient survival (P<0.01) are consistent with those reported in the first published safety analysis of the MORE study (8). These factors include poor ECOG performance status, markers of advanced disease (extra-hepatic metastases, elevated levels of carcinoembryonic antigen [CEA], increased extent of tumor-to-target liver involvement), and increased lines of chemotherapy. Poor liver function at baseline is also significantly associated with poor survival, as demonstrated by presence of ascites and the results of liver enzyme tests. Specifically, we found that abnormal levels of albumin, alkaline phosphatase, aspartate aminotransferase, and bilirubin at baseline are all associated with poor survival. Lung shunt fraction >10% and pre-treatment anemia (hemoglobin CTC grade ≥1) were also significantly associated with poor survival (Table 2 and Figures 1,2).
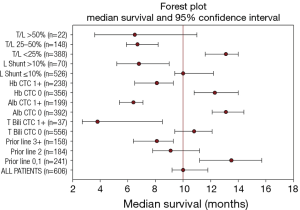
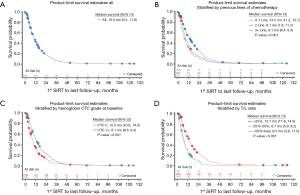
Full table
Subgroup analysis of long-term survivors
As of September 2016, 263 of 606 total patients were confirmed to have survived for at least 1 year following first 90Y-RE treatment. Subgroup analysis was performed of this set of long-term survivors with the goal of identifying predictors of long-term survival. As anticipated, baseline characteristics and treatment-related factors significantly associated with survival 1 year (P<0.01) overlapped with factors generally associated with survival outcomes (Table 2 and Table 3), including indicators of advanced disease, liver function, hemoglobin levels (anemia), and number of lines of prior chemotherapy.
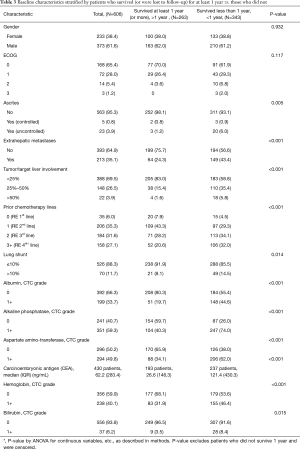
Full table
Discussion
The MORE study is the largest to date of metastatic colorectal cancer patients who received 90Y-RE as monotherapy after failed lines of systemic therapy. Initial analysis of this study examined safety and efficacy of 90Y-RE in this patient population. Subsequent analyses focused more closely upon baseline characteristics and factors associated with OS in these patients. Here we present an updated survival analysis from long-term follow-up of the MORE patients. Extending through September 2016, our analysis was initiated more than 14 years ago, and includes dates of death for 574 out of 606 patients, or 95% of our starting patient population.
Our results confirm that 90Y-RE treatment appears to offer survival benefits for patients with unresectable colorectal cancer liver metastases refractory to first-line chemotherapy. Patients treated with 90Y-RE in the second-line setting (after 1 line of failed chemotherapy) had a median survival time after 90Y-RE of 13.2 months (Table 2). This is similar to that of comparable patients treated in the second-line setting with the irinotecan-based FOLFIRI regimen alone (12.06 months) or combined with the antiangiogenic agent aflibercept (13.50 months) (9). It also compares favorably with survival times of metastatic colorectal cancer patients treated with second-line oxaliplatin or irinotecan-based chemotherapy alone (9.8 months median survival) or in combination with bevacizumab (median survival 11.2 months) (10). MORE patients treated with 90Y-RE as third-line treatment (i.e. after 2 failed lines of chemotherapy) had a median survival of 9.1 months, and patients treated with 90Y-RE as fourth-line therapy or higher (i.e. after 3 failed lines of chemotherapy or more) had a median survival time of 8.1 months (Table 2). These survival times compare favorably with similar patients treated with systemic therapies in the third-line setting or higher. For instance, metastatic colorectal cancer patients in the CONCUR trial were treated with the multikinase inhibitor regorafenib after at least 2 prior lines of failed therapy; these patients had a median survival of 8.8 months on regorafenib (6.3 months on placebo group) (19). Metastatic colorectal cancer patients treated with TAS-102, an oral agent combining trifluridine and tipiracil hydrochloride, after at least 2 prior lines of failed therapy had a median survival time of 7.1 months (5.3 months on placebo) (20). Overall, our results indicate that 90Y-RE treatment for metastatic colorectal cancer patients in the second-line setting or higher may offer survival benefits for appropriate patients (i.e. those with unresectable liver-dominant or liver-only disease).
Our results show that the number of prior lines of chemotherapy is a significant predictor of survival following 90Y-RE in our patient population. This confirms results from the first analysis of the MORE study (8). Our updated analysis also identified other factors significantly associated with survival, again consistent with previous analyses of the MORE data. These factors include high tumor burden in the liver, laboratory values indicative of poor liver function at baseline (e.g. total bilirubin and albumin levels), low levels of hemoglobin indicative of pre-treatment anemia, and lung shunt fraction greater than 10% (Table 2). Screening of such baseline factors may be used to optimize patient selection for 90Y-RE treatment. It has also been previously suggested that correction of anemia before 90Y-RE treatment (for instance, by blood transfusion or subcutaneous erythropoietic) may improve survival outcomes (13). Consistent with a previous report, we found no significant difference in survival outcomes between elderly patients ≥70 years and those younger (Table 2). This confirms our earlier conclusion that 90Y-RE is a safe and effective treatment for elderly patients with unresectable colorectal cancer liver metastases, and that age should not be a criteria for exclusion from this treatment (12).
90Y-RE has traditionally been administered as treatment for metastatic colorectal cancer liver metastases after failure of chemotherapies and other systemic therapies to control the disease (4-6). In 2017, based partly upon earlier analyses of the MORE study as well as other supporting studies, the National Comprehensive Cancer Network (NCCN) adopted new guidelines recommending 90Y-RE as a treatment option (category 2A evidence) for highly selected patients suffering from chemotherapy-resistant or chemotherapy-refractory metastatic colorectal cancer with predominant liver metastases (2017 NCCN guidelines in preparation). As of this moment, the great majority of metastatic colorectal cancer patients treated with 90Y-RE receive it as salvage therapy (i.e. after first receiving 2 or more failed lines of chemotherapy) (7). The patient population of the MORE study reflects this general practice; patients received a median of 2 (range, 0-6) lines of chemotherapy prior to 90Y-RE. Current research, however, has begun to focus upon the use of 90Y-RE in combination with chemotherapy as first-line treatment for colorectal cancer liver metastases. An early phase II trial demonstrated that addition of 90Y-RE to fluorouracil and leucovorin-based chemotherapy in patients with colorectal cancer liver metastases improved time to progressive disease as well as overall median survival (29.4 median months for patients treated with chemotherapy plus 90Y-RE versus 12.8 months for patients treated with chemotherapy alone, P=0.02) (21). In 2007, results from a phase I study demonstrated that 90Y-RE could be safely combined with oxaliplatin-based chemotherapy (22). Based upon such small-scale studies, 3 large-scale phase III trials have been initiated to examine the effect of 90Y-RE addition to oxaliplatin-based chemotherapy in treatment of metastatic colorectal cancer liver metastases in the first-line setting. A pooled analysis of survival outcomes from these 3 trials, the SIRFLOX, FOXFIRE, and FOXFIRE-Global clinical trials, will cover data from over 1000 patients. Survival outcomes are expected to be announced in 2017 (23). Data already available from the SIRFLOX trial has demonstrated that the addition of 90Y-RE to FOLFOX-based chemotherapy plus or minus bevacizumab significantly improved median progression-free survival (PFS) in the liver by 7.9 months (23).
These studies on the role of 90Y-RE in combination with chemotherapy in the first-line treatment of metastatic colorectal cancer have the potential to change the current treatment paradigm. Research is also underway on the use of 90Y-RE in combination with other chemotherapy regimens and systemic therapies, including the use of targeted agents, in other settings. Currently, a phase III trial is underway to compare the effect of 90Y-RE treatment plus modified LV5FU2 chemotherapy plus or minus targeted biologicals (bevacizumab, cetuximab, or panitumumab according to previous use) versus chemotherapy plus or minus targeted agents alone in metastatic colorectal cancer first controlled with induction chemotherapy (clinical trials.gov identifier NCT01895257). A phase I trial has been designed to evaluate the safety of 90Y-RE in combination with the cytotoxic drug TAS-102 in the treatment of chemotherapy refractory metastatic colorectal cancer (clinicaltrials.gov identifier NCT02602327). There is also a published case study on the use of 90Y-RE in combination with FOLFIRI chemotherapy and the anti-angiogenic agent aflibercept in treatment of metastatic colorectal cancer liver metastases. The authors of this case study report that the patient’s liver metastases showed partial response, and conclude that this combination therapy was both safe and efficacious in this patient (24).
Such ongoing studies may open up new avenues for the application of 90Y-RE in the treatment of metastatic colorectal cancer liver metastases. As of this moment, however, the majority of patients with metastatic colorectal cancer treated with 90Y-RE are treated with 90Y-RE as monotherapy after multiple lines of failed chemotherapy. The MORE study currently remains the most comprehensive data set involving 90Y-RE treatment in patients after prior failed lines of chemotherapy. Our updated analysis confirms that 90Y-RE treatment provides a meaningful response and survival advantage for MORE patients across all ages and across diverse community and academic centers in the U.S. Overall, this analysis offers reliable information to support 90Y-RE treatment in the eligible metastatic colorectal cancer patients typically seen in most centers, and should help enable proper selection as future patients are treated.
Conclusions
Our updated analysis of the MORE study confirms that 90Y-RE treatment offers favorable survival benefits for patients with unresectable metastatic colorectal cancer patients, even among patients who had received 3 or more prior lines of chemotherapy. Our analysis also confirms earlier reported prognostic factors for survival after 90Y-RE, including number of lines of prior chemotherapy, markers of advanced disease, poor liver function at baseline, pre-treatment anemia, and lung shunt fraction >10%.
Acknowledgements
The authors thank Vanessa Fogg, PhD, of Eubio Medical Communications, LLC for providing medical editorial support of manuscript development and Mark Van Buskirk of Data Reduction who provided statistical support, both funded by Sirtex Medical, Inc.
Funding: This was an investigator-initiated study funded by Sirtex Medical Limited, Sydney, Australia through an educational grant awarded to Dr. Kennedy, Sarah Cannon Research Institute.
Footnote
Conflicts of Interest: Received research grants from Sirtex Medical (AKennedy, MS, AD); consultant to Sirtex Medical (AD, EW); participant in speakers’ bureau for Sirtex Medical (DC, MC, CN); owns stock holdings in Sirtex Medical (AD, SR). SR has also served as a consultant for Surefire Medical, XL Sci-Tech, and Guerbet.
Ethical Statement: Data at each site were collected from source documentation by an independent contract research organization and each site was granted institutional review board exemptions prior to data collection.
References
- American Cancer Society. Cancer Facts & Figures 2016. Cancer Facts Fig 2016;2016:1-9.
- De Greef K, Rolfo C, Russo A, et al. Multisciplinary management of patients with liver metastasis from colorectal cancer. World J. Gastroenterol 2016;22:7215-25. [Crossref] [PubMed]
- Dhir M, Jones HL, Shuai Y, et al. Hepatic arterial infusion in combination with modern systemic chemotherapy is associated with improved survival compared with modern systemic chemotherapy alone in patients with isolated unresectable colorectal liver metastases: A case-control study. Ann Surg Oncol 2017;24:150-8. [Crossref] [PubMed]
- Gruber-Rouh T, Marko C, Thalhammer A, et al. Current strategies in interventional oncology of colorectal liver metastases. Br J Radiol 2016.20151060. [Crossref] [PubMed]
- National Comprehensive Cancer Network Guidelines: Colon Cancer Version 1.2017--Nov 23, 2016. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- National Comprehensive Cancer Network Guidelines: Rectal Cancer Version 2.2017--Dec 22, 2016. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Kennedy A. Radioembolization of hepatic tumors. J Gastrointest Oncol 2014;5:178-89. [PubMed]
- Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastrointest Oncol 2015;6:134-42. [PubMed]
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499-506. [Crossref] [PubMed]
- Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol 2013;14:29-37. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Kennedy AS, Ball DS, Cohen SJ, et al. Safety and efficacy of radioembolization in elderly (≥70 years) and younger patients with unresectable liver-dominant colorectal cancer. Clin Colorectal Cancer 2016;15:141-151.e6. [Crossref] [PubMed]
- Kennedy AS, Ball D, Cohen SJ, et al. Baseline hemoglobin and liver function predict tolerability and overall survival of patients receiving radioembolization for chemotherapy-refactory metastatic colorectal cancer. J Gastrointest Oncol 2017;8:70-80. [Crossref] [PubMed]
- Narsinh KH, Buskirk M, Van , Kennedy AS, et al. Hepatopulmonary shunting: a prognostic indicator of survival in patients with metastatic colorectal adenocarcinoma treated with 90 Y Radioembolization. Radiology 2017;282:281-8. [Crossref] [PubMed]
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: A Consensus Panel Report from the Radioembolization Brachytherapy Oncology Consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. [Crossref] [PubMed]
- Coldwell DM, Sewell PE. The expanding role of interventional radiology in the supportive care of the oncology patient: From diagnosis to therapy. Semin Oncol 2005;32:169-73. [Crossref] [PubMed]
- Salem R, Thurston KG, Carr BI, Goin JE, Geschwind J-FH. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. J Vasc Interv Radiol 2002;13:S223-S229. [Crossref] [PubMed]
- Common terminology criteria for adverse events (CTCAE) version 4.03. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619-29. [Crossref] [PubMed]
- Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized Trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909-19. [Crossref] [PubMed]
- Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres® plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88:78-85. [Crossref] [PubMed]
- Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol 2007;25:1099-106. [Crossref] [PubMed]
- van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: Randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol 2016;34:1723-1731. [Crossref] [PubMed]
- De Souza A, Daly KP, Yoo J, et al. Safety and efficacy of combined yttrium 90 resin radioembolization with aflibercept and FOLFIRI in a patient with metastatic colorectal cancer. Case Rep Oncol Med 2015;2015:461823. [Crossref] [PubMed]


