Comparison of efficacy and toxicity of FOLFIRINOX and gemcitabine with nab-paclitaxel in unresectable pancreatic cancer
Introduction
Prognosis of unresectable pancreatic cancer is poor with a 5-year survival rate not exceeding 6% (1). The nucleoside analog gemcitabine was standard first-line chemotherapy for metastatic pancreatic cancer (2) until the three-drug regimen irinotecan, oxaliplatin and leucovorin-modulated fluorouracil (FOLFIRINOX) improved the prognosis of patients in 2011 (3). In 2013, gemcitabine and nanoparticle albumin-bound paclitaxel (GnP) (nab-PTX) were also shown to increase survival of patients with metastatic pancreatic cancer (4). However, these two phase III randomized trials did not include Japanese patients. In Japan, we can treat unresectable, including locally advanced, pancreatic cancer with FOLFIRINOX and GnP, on the basis of two single-armed phase II trials in Japanese patients with metastatic pancreatic cancer (5,6). Those trials showed that the rates of adverse events in Japanese patients were higher than those of previous phase III trials. However, no study has investigated which regimen is better for unresectable pancreatic cancer.
In Japan, we have treated patients with unresectable locally advanced or metastatic pancreatic cancer with FOLFIRINOX from December 2013, and with GnP from December 2014 using the Japanese national public health insurance. In the present study, we compared the efficacy and safety of first-line chemotherapy with FOLFIRINOX or GnP in Japanese patients with unresectable pancreatic cancer.
Methods
Patients
We retrospectively analyzed the medical records of all patients with unresectable locally advanced or metastatic pancreatic cancer treated with FOLFIRINOX or GnP as first-line chemotherapy between December 2013 and September 2015 at Hokkaido University Hospital.
Study design
This study was a retrospective cohort, single-institution analysis. Its objectives were to compare the efficacy and safety of FOLFIRINOX with those of GnP. The study was approved by the Institutional Review Board of Hokkaido University Hospital (the registration number is 015-0335), and conducted according to the Declaration of Helsinki.
Treatment
FOLFIRINOX consisted of 85 mg/m2 of oxaliplatin administered over 2 h, followed by 180 mg/m2 of irinotecan and 200 mg/m2 of L-leucovorin administered over 90 min. This was followed by 400 mg/m2 of fluorouracil as a bolus and 2.4 g/m2 of fluorouracil as a 46-h continuous infusion. All patients were administered 0.75 mg of palonosetron, 9.9 mg of dexamethasone and 125 mg of aprepitant before chemotherapy, followed by 80 mg of aprepitant on the following 2 days to prevent chemotherapy-induced nausea and vomiting (CINV). Treatment cycles were repeated every 2 weeks until tumor progression or intolerable toxicity occurred.
GnP consisted of 125 mg/m2 nab-PTX over 30 min, followed by 1 g/m2 gemcitabine over 30 min, on days 1, 8 and 15. Treatment cycles were repeated every 4 weeks until tumor progression or intolerable toxicity occurred. The patients were administered 0.75 mg of palonosetron and/or 9.9 mg of dexamethasone before chemotherapy to prevent CINV if needed.
Statistical analysis
Patient age and tumor markers were compared using Mann–Whitney U test, and sex, performance status, presence or absence of biliary stent, and disease status (metastatic, recurrent or locally advanced) were compared using Fisher’s exact test. Progression-free survival (PFS) was measured from the first day of chemotherapy to the time of disease progression or last follow-up. Overall survival (OS) was measured from the first day of chemotherapy to the time of death or last follow-up. The period of follow-up was estimated using the reverse Kaplan-Meier method. OS and PFS were calculated using the Kaplan–Meier method and were compared using the log-rank test. The hazard ratio (HR) was calculated using univariate Cox proportional hazard regression modeling. Statistical analyses were carried out using IBM SPSS Statistics version 23. Response evaluation was based on the revised Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and was compared using Fisher’s exact test. Adverse events were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Results
Patient characteristics
We identified 16 patients treated with FOLFIRINOX and 22 with GnP as first-line chemotherapy at our institution between December 2013 and September 2015. The median age was 63 years (range, 49–71 years) in the FOLFIRINOX group and 66.5 years (range, 49–78 years) in the GnP group. Seven patients in the FOLFIRINOX group and four in the GnP group had locally advanced unresectable pancreatic cancer. Eleven patients in the FOLFIRINOX group and seven in the GnP group were assessed as WHO performance status (PS) 1 and the other patients were PS 0 (P=0.047). There were a larger number of patients with metastatic pancreatic cancer in the GnP group than in the FOLFIRINOX group (P<0.001). Other patient characteristics are listed in Table 1.
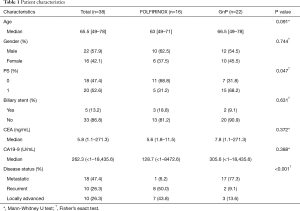
Full table
The median number of treatment cycles administered was six in the FOLFIRINOX group (range, 1–12) and three in the GnP group (range, 1–6). The median relative dose intensities of fluorouracil as a bolus infusion, fluorouracil as a 46-h continuous infusion, irinotecan, oxaliplatin, gemcitabine and nab-PTX were 19.9%, 87.2%, 73.8%, 85.1%, 78.0% and 70.4%, respectively.
Efficacy
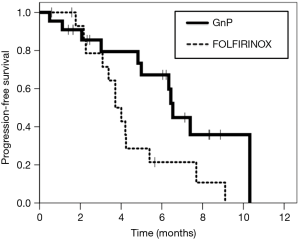
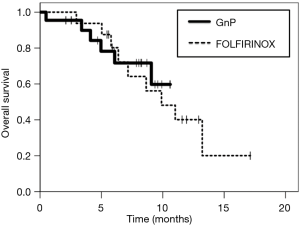
The median follow-up period was 11.9 and 8.3 months in the FOLFIRINOX and GnP groups, respectively. The objective response rate was 6.3% (one of 16 patients) in the FOLFIRINOX group and 40.9% (nine of 22 patients) in the GnP group. Disease control rate was 56.3% (nine of 16 patients) in the FOLFIRINOX group and 86.4% (19 of 22 patients) in the GnP Group. The median PFS was 3.7 months [95% confidence interval (CI), 3.0–4.5 months] in the FOLFIRINOX group and 6.5 months (95% CI, 6.2–6.9 months) in the GnP group (HR, 0.41; 95% CI, 0.18–0.95; P=0.031) (Figure 1). The median OS was 9.9 months (95% CI, 5.9–13.9 months) in the FOLFIRINOX group and not reached in the GnP group (Figure 2). The efficacy in the two groups is summarized in Table 2.
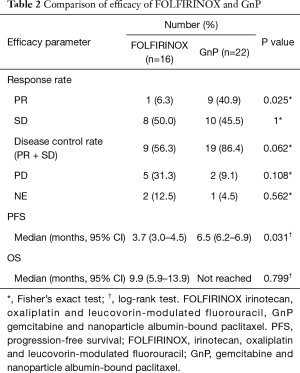
Full table
Second-line therapy
We administered second-line therapy to 15 patients in the FOLFIRINOX group and three in the GnP group. The second-line regimens were gemcitabine monotherapy (seven patients), GnP (four patients), other gemcitabine-based doublet (one patient), S-1 (one patient), radiotherapy (two patients) in the FOLFIRINOX group, and FOLFIRINOX (two patients) and S-1 (one patient) in the GnP group.
Adverse events
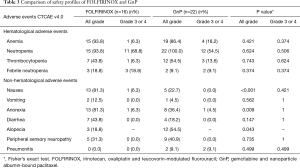
No patients died from treatment-related causes in either group. The most common treatment-related grade 3 or 4 adverse event was neutropenia, which occurred in 68.8% and 54.5% of patients in the FOLFIRINOX and GnP groups, respectively. There was no significant difference in the incidence of grade 3 or 4 neutropenia, febrile neutropenia, thrombocytopenia, sensory neuropathy, nausea, and anorexia between the two groups. The common treatment-related grade 3 or 4 adverse events in the two groups are summarized in Table 3.
Full table
Discussion
Gemcitabine monotherapy has been the standard regimen for patients with metastatic or unresectable locally advanced pancreatic cancer since 1997 (2), but the 1-year survival rate of patients with metastatic disease is only 17–23% (2,3,6). A phase III trial of erlotinib plus gemcitabine compared with gemcitabine alone demonstrated significantly improved survival in advanced pancreatic cancer (median OS 6.24 vs. 5.91 months) (7). A phase III trial of FOLFIRINOX versus gemcitabine showed significant improvement for patients with metastatic pancreatic cancer (median OS 11.1 vs. 6.8 months) (3). The GnP regimen was also found to improve OS for patients with pancreatic cancer in a phase III trial (median OS 8.5 vs. 6.7 months) (4).
FOLFIRINOX and GnP regimens yield better prognosis than gemcitabine monotherapy for metastatic pancreatic cancer, however, there has been no phase III study for unresectable locally advanced pancreatic cancer with these two regimens. FOLFIRINOX and GnP regimens are widely used for advanced pancreatic cancer, based on the clinical benefit of two phase III studies (3). There has been no comparative trial of these two regimens, and our study is believed to be the first comparison of the two regimens.
In our study, the GnP regimen showed a similar clinical outcome to a previous phase III study (4), but the PFS in the FOLFIRINOX group was shorter than in another previous phase III study (3). Those two studies of metastatic pancreatic cancer and our study included unresectable locally advanced cancer, and FOLFIRINOX is reported to have a clinical benefit in locally advanced pancreatic cancer as well as metastatic pancreatic cancer (8,9). However, none of the previous prospective trials included Japanese patients. It was previously reported that adverse events were more frequent in Japanese patients using FOLFIRINOX (5). This means that the FOLFIRINOX regimen may be too toxic for Japanese patients. In our study, the relative dose intensity in the FOLFIRINOX group was similar to that previously reported, but resulted in poor outcome in the FOLFIRINOX group. In our study, almost all patients in the FOLFIRINOX group received second-line chemotherapy compared with only a few patients in the GnP group. This might explain the similar OS between the two treatment groups, even if there was significantly poor PFS in the FOLFIRINOX group. We need further studies of the efficacy and safety of FOLFIRINOX in Japanese patients with pancreatic cancer. Currently, a multicenter prospective trial of dose-modified FOLFIRINOX in patients with advanced pancreatic cancer is in progress in Japan, and we await the results for efficacy and safety. In our study, the response rate in the GnP group was almost as high as that previously reported. GnP is one of the neoadjuvant chemotherapy regimens in patients with borderline resectable locally advanced pancreatic cancer.
Our study was limited by its small sample size, retrospective analysis, and being conducted in a single institution. However, we suggest that the GnP regimen yields a better clinical outcome for more patients with pancreatic cancer with less toxicity than the FOLFIRINOX regimen does. Our results should be confirmed by additional, prospective randomized trials.
Acknowledgements
We would like to thank the patients, their families, and the staff members of the Departments of Gastroenterology and Hepatology and Cancer Center of Hokkaido University Hospital. Additionally, we would like to thank Edanz Group Ltd. (www.edanzediting.co.jp) for the English language review.
Footnote
Conflicts of Interest: Y Komatsu received honoraria from Taiho Pharmaceutical Co. Ltd., Yakult Pharmaceutical Industry Co. Ltd., Daiichi Sankyo Ltd. and Bristol-Myers Squibb. N Sakamoto received honoraria from Bristol-Myers Squibb, MSD, Gilead Sciences, and Otuka. The other authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Ethical statement: The study was approved by the Institutional Review Board of Hokkaido University Hospital (No. 015-0335), and conducted according to the Declaration of Helsinki. And written informed consent was obtained from all patients.
References
- Conroy T, Bachet JB, Ayav A, et al. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer 2016;57:10-22. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Okusaka T, Ikeda M, Fukutomi A, et al. Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci 2014;105:1321-6. [Crossref] [PubMed]
- Ueno H, Ikeda M, Ueno M, et al. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 2016;77:595-603. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Gunturu KS, Yao X, Cong X, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol 2013;30:361. [Crossref] [PubMed]
- Moorcraft SY, Khan K, Peckitt C, et al. FOLFIRINOX for locally advanced or metastatic pancreatic ductal adenocarcinoma: the Royal Marsden experience. Clin Colorectal Cancer 2014;13:232-8. [Crossref] [PubMed]


