Gemcitabine and capecitabine for advanced biliary cancer
Introduction
Biliary cancer is comprised of intrahepatic cholangiocarcinoma (IHCC), extrahepatic cholangiocarcinoma (EHCC) and gallbladder carcinoma (GBC). Depending on the type, the incidence of biliary cancer in the US ranges from 0.6–1.5 persons per 100,000 and has been increasing (1-4). Although less common than other malignancies, patients often present with locally advanced or metastatic disease, which is reflective of their aggressive biology, late stage at diagnosis and poor prognosis with an average 5-year survival rate of 5% (5-7). There are limited therapeutic options for advanced biliary cancer (ABC) as outlined by the guidelines of the National Comprehensive Cancer Network (NCCN) (8). Surgical resection is a potential option for patients with locally advanced, non-metastatic ABC. This may be followed by adjuvant chemotherapy or radiation.
There have been several trials supporting the use of chemotherapy in patients with ABC. The phase II study ABC-01 conducted in the United Kingdom demonstrated that the combination of gemcitabine with cisplatin (gem-cis) was superior to gemcitabine alone (9). The larger phase III randomized trial, ABC-02, showed a median overall survival (OS) of 11.7 months (mo) in the gem-cis group compared to the group that received gemcitabine alone [median OS of 8.1 mo, 95% confidence intervals (CI): 0.52–0.80, P<0.001] (10). Patients with locally advanced cancer had increased benefit from gem-cis with a hazard ratio (HR) of 0.47 (95% CI: 0.29–0.74) compared to metastatic disease (HR =0.74, 95% CI: 0.57–0.95) (11). However, approximately 70% of patients experienced a grade 3 or 4 toxicity (10).
Authors of this current study previously reported results from a small phase II study using gemcitabine with capecitabine (gem-cap) for 12 patients with ABC, showing an overall response rate of 58%, a median time to tumor progression of 9.0 mo and a median OS of 14.0 mo (12). In this small cohort, 75% experienced a grade 3/4 toxicity, with the most common being a hematologic abnormality (3 with neutropenia and 1 with thrombocytopenia). The gem-cap regimen has continued to be used in the first line for ABC at our institution. The purpose of this study was therefore to provide an updated analysis of the survival outcomes and toxicities of gem-cap for ABC.
Methods
Patients
This is a single institution, retrospective review of patients with ABC, both locally advanced and metastatic, from 2005–2015. The study was approved by the Institutional Review Board (IRB) at the Roswell Park Cancer Institute (DTA BDR 058715-20150505). A query of the electronic medical record (EMR) was performed using the following International Statistical Classification of Diseases (ICD-O-3) codes: 155.0, 155.1, 156.0, 156.1, 156.9 and V10.09.
Patients with prior therapies such as biliary tract stenting, surgical resection (partial or complete) or palliative surgeries were included. However, patients who were treated elsewhere or received prior chemotherapy (in either an adjuvant or systemic setting) were excluded. Thus, the primary analysis was performed on patients who had received gem-cap as the first line chemotherapy. Patients who progressed on gem-cap and received second or third line therapies, including alternate chemotherapy regimens or immunotherapies, were included.
At Roswell Park, gemcitabine is typically administered at a dose of 1,000 mg/m2 intravenously over 30 minutes on days 1 and 8. Capecitabine is administered orally at 650 mg/m2 twice daily for 14 days. Each cycle is repeated every 21 days. Dose modification for both gemcitabine and capecitabine has previously been described in our previous phase II trial (12).
Patient demographics, tumor characteristics and treatment variables were collected. The primary outcomes included progression-free survival (PFS) and OS. PFS was defined as the time from gem-cap until progression, death, or last follow-up. Tumor responses and progression were measured by computed tomography (CT) scans using standard Response Evaluation Criteria in Solid Tumors (RECIST) criteria. OS was defined as the time from gem-cap until death or last follow-up. The secondary outcome was assessment of grade 3 or 4 toxicities. All patients were evaluated for toxicity from the time of their first treatment with gem-cap. Toxicity was assessed prior to each dose of chemotherapy throughout treatment using the National Cancer Institute Common Toxicity Criteria (NCI CTC) version 2.0.
Statistical analysis
Patient demographic, clinical, and treatment characteristics were reported using means and standard deviations for continuous variables, and using frequencies and relative frequencies for categorical variables. Comparisons were made using the Mann-Whitney U or Kruskal-Wallis tests as appropriate for continuous variables, and using Fisher’s exact test for categorical variables. The time to event outcomes, OS and PFS, were reported by cohort (locally advanced versus metastatic disease and tumor location including IHCC, EHCC and GBC) using standard Kaplan-Meier methods with estimates of median survival and 1- and 3-year survival rates obtained with 95% CI. Comparisons were made using the log-rank test. All analyses were conducted in SAS v9.4 (Cary, NC, USA) at a significance level of 0.05.
Results
Data query based on the included ICD-O-3 codes yielded 1,230 patients. After applying the remaining inclusion and exclusion criteria, 372 patients were identified of which 227 (61.0%) patients received chemotherapy at our institution. Of these, 153 (67.4%) patients received gem-cap, and 129 (56.8%) were treated as the first line. Figure 1 summarizes the sequential inclusion and exclusion criteria used to obtain the final study cohort. A total of 42 patients (32.6%) had locally advanced disease, and 87 (67.4%) had metastatic disease. Of the patients with locally advanced disease, 24 (57.1%, 24/42) were treated with gem-cap in the adjuvant setting and the remaining 18 (42.9%) had unresectable, non-metastatic disease who received gem-cap as first line systemic therapy.
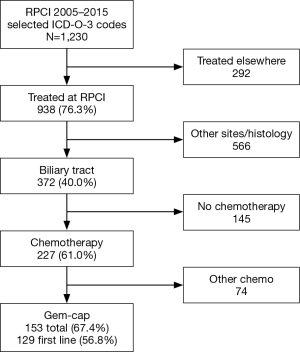
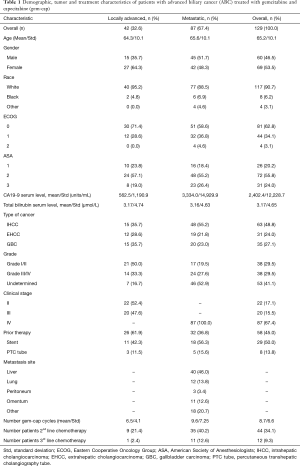
Table 1 shows the patient demographics, tumor characteristics and treatments grouped by locally advanced and metastatic disease. The overall distribution of tumor location was 48.9% IHCC, 24.0% EHCC and 27.1% GBC. The average number of cycles of gem-cap was 6.0. Overall, 45.0% of patients had prior therapy, mainly consisting of bile duct stenting either through endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC).
Full table
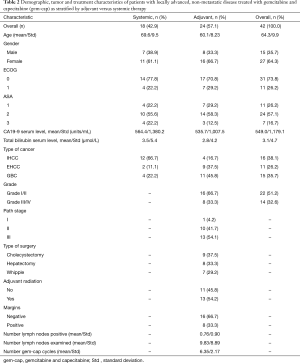
Table 2 shows the characteristics for patients with locally advanced disease grouped by gem-cap used in the adjuvant versus systemic setting. Patients who received gem-cap as adjuvant therapy were more likely to have a GBC primary (45.8%), whereas patients who received gem-cap as systemic therapy were more likely to have an IHCC primary (68.4%). The types of surgeries performed are also shown in Table 2, with a negative margin achieved in 66.7% of cases.
Full table
Regarding radiation, 54.2% of patients received adjuvant radiation following surgery. Overall, a total of 44 patients (34.1%) received second line chemotherapy, the most common of which was FOLFOX. Twelve patients (9.3%) received third line chemotherapy.
Figure 2 shows the PFS and OS for the overall cohort grouped by locally advanced versus metastatic disease and by tumor location. The median PFS for the entire cohort was 8.0 mo (95% CI: 6.0–9.3), whereas the median OS was 13.0 mo (95% CI: 10.7–17.4). As shown, patients with locally advanced, non-metastatic disease had superior outcomes to those with metastatic disease. There were no statistically significant differences in survival outcomes when analyzed by tumor site. Of the patients with locally advanced, non-metastatic ABC who received gem-cap as adjuvant therapy, the median PFS was 25.3 mo (range, 8.8–96.9 mo) and approximately 40% achieved durable responses at 3 years. Similarly in this subgroup of patients, the median OS was 27.4 mo with a 3-year OS rate of 49%.
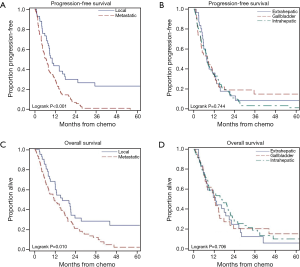
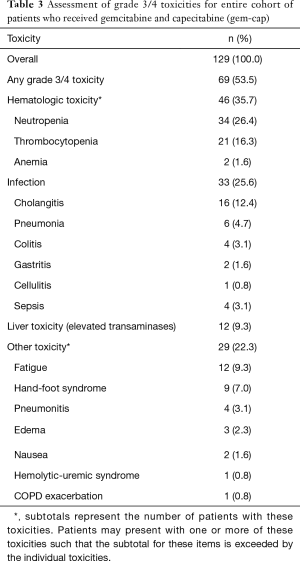
Table 3 shows the analysis of grade 3/4 toxicities. Overall 53.5% (69/129) experienced a grade 3/4 toxicity. The most common toxicity (35.7%) was hematologic (neutropenia or thrombocytopenia) followed by infection (25.6%). Other adverse events included elevated liver function tests (9.3%) and non-hematologic events (22.3%), including fatigue (9.3%) and hand-foot syndrome (7.0%).
Full table
Discussion
Several studies have shown the benefit of gem-cap for advanced cancer, including those of the biliary tract (13-19). A systemic review of 13 single-arm phase II trials confirmed the benefits of gemcitabine in combination with a fluoropyrimidine (5-FU or capecitabine) for ABC (20). We have previously reported that gem-cap is beneficial for ABC with an overall response rate of 58%, a PFS of 9.0 mo and median OS of 14 mo (12). In this updated analysis, we showed similar survival outcomes. The gem-cap regimen was fairly well tolerated with a grade 3/4 toxicity rate of 53.5% with the main toxicity being hematologic aberrancies, including neutropenia and thrombocytopenia. However, the toxicity rate observed in this current study was markedly lower than the previously reported 75% grade 3/4 rate in our previous small phase II trial, which consisted of only 12 patients. Therefore, the risk to benefit ratio appears to have improved significantly with further use of this treatment regimen at our institution.
Since our prior study and others, gem-cap has been a recognized adjuvant and systemic treatment option for patients with ABC (8). More recently, however, results of the ABC-02 trial has shown in a randomized phase III clinical trial that gem-cis, as compared to gemcitabine alone, had a tumor control rate of 81.4%, median PFS of 8.0 mo, median OS of 11.7 mo and adverse event rate of 70% (10). The ABC-02 trial constitutes level 1 evidence supporting gem-cis for ABC. In this study, we sought to update our analysis of gem-cap and compare these results to those reported in the ABC-02 trial, recognizing that a direct statistical comparison was not possible given the differences in the fundamental design of each study and instead used the ABC-02 trial results as a historical comparison.
Our updated analysis had a longer median follow-up compared to the phase II trial performed by Iyer et al. (45.1 vs. 18.2 mo) (12), and showed a PFS of 8.0 mo and OS of 13.0 mo. These results were consistent with the prior Iyer trial. With a longer follow-up and a substantially increased number of patients available in this analysis compared to the Iyer phase II trial, a more favorable adverse event rate was found (53.5% vs. 75%). Herein highlights the potential clinical benefit of gem-cap as compared to gem-cis, as the former may be better tolerated than gem-cis in terms of side-effect profile (70% in the ABC-02 trial). Capecitabine also has the advantage of oral dosing, which may facilitate drug delivery and patient compliance with therapy (21,22). Although quality of life (QOL) measurement was not performed in this study, the favorable side-effect profile had been reflected in improved or maintained QOL as reported previously (12).
Although the toxicity of gem-cap appeared to be favorable by historical comparison to gem-cis, these regimens offer similar benefits in terms of PFS and OS. Interestingly for patients with surgery and adjuvant gem-cap, durable long-term responses were found. However for patients with unresectable, locally advanced disease or metastatic disease, current treatment options including gem-cap or gem-cis are still lacking in terms of long-term survival. The paucity of effective, durable therapies for advanced disease is reflected in Figure 2 showing minimal (12%) long-term survival at 3 years compared to the 29% OS in patients with locally advanced and potentially resectable disease. Moreover, at 3 years the majority of patients with metastatic disease have progressed, with only 1% having PFS.
Therefore, the need for more effective systemic or regional therapies for local ABC is pressing. There are some studies combining chemotherapy (gem-cis) with immunotherapies or molecular therapies to determine if this combination offers enhanced tumor responses. Results of a multi-institutional phase II trial including the Roswell Park Cancer Institute investigated the addition of bevacizumab (BV) to gem-cap in ABC (23). Survival results for patients treated with gem-cap and BV (median PFS =8.1 mo and median OS =11.3 mo) were similar to those treated with gem-cap only. The phase II ABC-03 trial combined cediranib, an oral vascular endothelial growth factor receptor (VEGFR) inhibitor with gem-cis (NCT00939848). Approximately 20% of the patients enrolled in this trial had local ABC. Initial results reported at the 2014 ASCO meeting showed that the addition of cediranib resulted in a trend for longer OS in the cediranib arm (14.1 mo, 95% CI: 10.2–16.0) as compared to gem-cis plus placebo (11.9 mo, 95% CI: 9.2–13.4, P=0.19). The phase II ABC-04 trial (UK CRUKE/10/036) combined gem-cis with selumetinib, a mitogen extracellular signal-regulated kinase (MEK) inhibitor involved in disruption of the mitogen-activated protein (MAP) kinase pathway. Results from this study are anticipated. Indeed, further study into more effective, long-term therapies is needed for ABC.
We recognize that there are important limitations to our updated analysis. As a retrospective study, there is the potential for incomplete capture of all patients with local ABC treated with gem-cap. Missing data and recall bias are also potential shortcomings of the study. Most importantly, direct comparison of this retrospective study with previous prospective trials, both the Iyer phase II and the ABC-02 phase III trial, is challenging as the differences in methodology limit any comparative statistical analysis. Therefore, comparison of this study with others can only be performed in a historical fashion. Nonetheless, the similar survival outcomes and favorable toxicity of gem-cap were clearly observed in this analysis and were consistent with other studies.
In conclusion, this updated analysis has shown that gem-cap has similar benefit as gem-cis in the treatment of ABC. In the setting of a more favorable adverse event profile, gem-cap potentially offers better tolerability than gem-cis. Capecitabine also offers the advantage of oral dosing, thus facilitating drug delivery and patient compliance. Prospective comparison of these regimens is therefore warranted.
Acknowledgements
Funding: This research was supported, in part, by the NCI Cancer Center Support Grant to Roswell Park Cancer Institute (CA016056) for the Biostatistics Shared Resource.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board (IRB) at the Roswell Park Cancer Institute (DTA BDR 058715-20150505) and written informed consent was obtained from all patients.
References
- McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2006;15:1198-203. [Crossref] [PubMed]
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353-7. [Crossref] [PubMed]
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99-109. [PubMed]
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [Crossref] [PubMed]
- Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-17; discussion 517-9. [Crossref] [PubMed]
- Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485-9. [Crossref] [PubMed]
- de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. N Engl J Med 1999;341:1368-78. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Hepatobiliary Cancers. (Version 2.2016). Accessed November 5, 2016. Available online: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- Valle JW, Wasan H, Johnson P, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer 2009;101:621-7. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Fabozzi M, Allieta R, Brachet Contul R, et al. Comparison of short- and medium-term results between laparoscopically assisted and totally laparoscopic right hemicolectomy: a case-control study. Surg Endosc 2010;24:2085-91. [Crossref] [PubMed]
- Iyer RV, Gibbs J, Kuvshinoff B, et al. A phase II study of gemcitabine and capecitabine in advanced cholangiocarcinoma and carcinoma of the gallbladder: a single-institution prospective study. Ann Surg Oncol 2007;14:3202-9. [Crossref] [PubMed]
- Schilsky RL, Bertucci D, Vogelzang NJ, et al. Dose-escalating study of capecitabine plus gemcitabine combination therapy in patients with advanced cancer. J Clin Oncol 2002;20:582-7. [Crossref] [PubMed]
- Santini D, Virzi V, Vincenzi B, et al. A phase I trial of fixed dose rate gemcitabine plus capecitabine in metastatic cancer patients. Ann Oncol 2007;18:576-80. [Crossref] [PubMed]
- Mani S, Vogelzang NJ, Bertucci D, et al. Phase I study to evaluate multiple regimens of intravenous 5-fluorouracil administered in combination with weekly gemcitabine in patients with advanced solid tumors: a potential broadly active regimen for advanced solid tumor malignancies. Cancer 2001;92:1567-76. [Crossref] [PubMed]
- Gebbia V, Giuliani F, Maiello E, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol 2001;19:4089-91. [Crossref] [PubMed]
- Giuliani F, Gebbia V, Maiello E, et al. Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a multicenter phase II study of the Gruppo Oncologico dell'Italia Meridionale (GOIM). Ann Oncol 2006;17 Suppl 7:vii73-7. [Crossref] [PubMed]
- Knox JJ, Hedley D, Oza A, et al. Gemcitabine concurrent with continuous infusional 5-fluorouracil in advanced biliary cancers: a review of the Princess Margaret Hospital experience. Ann Oncol 2004;15:770-4. [Crossref] [PubMed]
- Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 2005;23:2332-8. [Crossref] [PubMed]
- Dingle BH, Rumble RB, Brouwers MC. The role of gemcitabine in the treatment of cholangiocarcinoma and gallbladder cancer: a systematic review. Can J Gastroenterol 2005;19:711-6. [Crossref] [PubMed]
- Bhattacharya D, Easthall C, Willoughby KA, et al. Capecitabine non-adherence: exploration of magnitude, nature and contributing factors. J Oncol Pharm Pract 2012;18:333-42. [Crossref] [PubMed]
- Patel K, Foster NR, Farrell A, et al. Oral cancer chemotherapy adherence and adherence assessment tools: a report from North Central Cancer Group Trial N0747 and a systematic review of the literature. J Cancer Educ 2013;28:770-6. [Crossref] [PubMed]
- Iyer RV, Pokuri VK, Groman A, et al. A Multicenter Phase II Study of Gemcitabine, Capecitabine, and Bevacizumab for Locally Advanced or Metastatic Biliary Tract Cancer. Am J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]


