Clinicopathologic study of neuroendocrine tumors of gastroenteropancreatic tract: a single institutional experience
Introduction
Neuroendocrine tumors (NET) of the gastroenteropancreatic (GEP) system are epithelial neoplasm with predominantly neuroendocrine differentiation and originate from diffuse endocrine system located in the gastrointestinal (GI) tract and in the pancreas (1). It represents 2% of all GI tumors. This is a heterogeneous group of tumors which present with variety of clinical symptoms. They can be functional or nonfunctional. All the NET have malignant potential and the malignant potential further depends on tumor site, degree of differentiation and extension of the tumor. GEP tumors are heterogeneous tumors and it is difficult to predict their behavior and prognosis. It has been seen that incidence of NETs are steadily increasing. It is important to publish the clinical and pathologic details of these tumors to better understand the variations with respect to location, grading and behavior. However there are very few dedicated studies on the occurrence and grading of these tumors. Their behavior is better in comparison to GI conventional adenocarcinomas (2). In the present study we have tried to compile the clinicopathological profile of the NETs which were diagnosed on biopsy or resection according to WHO 2010 criteria (3). We have analyzed the immunohistochemical (IHC) features along with Ki67 index and also tried to correlate the grade of the tumor with Ki67 labeling index.
Methods
All the cases of gastroenteropancreatic neuroendocrine tumors (GEPNET) diagnosed at Department of Pathology, Nizam’s Institute of Medical Sciences from January 2012 to June 2015 were analyzed. The demographic data and clinical details were retrieved from the medical records. The location, size, color and consistency of the tumors were examined in all the resected specimens on gross examination. The hematoxylin and eosin (H & E) stained sections were reviewed and histomorphological features including the cellular arrangement and cell morphology pertaining to the various sites were analyzed in all the cases. The diagnosis of neuroendocrine tumor was made on both biopsies as well as resected specimens. The classification and grading of these tumors were done according to WHO 2010 classification (3). IHC was done with chromogranin, synaptophysin and pancytokeratin in 40, 24 and 13 cases respectively. Ki67 was done in all cases. All the primary antibodies (chromogranin, synaptophysin, pancytokeratin and Ki67) were ready to use, mouse monoclonal antibodies supplied by Bio Genex, CA. IHC were performed on fully automated immunostainer (i6000; Bio Genex) by using poly horse radish peroxide (HRP) technique.
Statistical analysis
Socio demographic data features and characteristics of tumors were expressed as number, percentage and mean value. Spearman correlation analysis was used to assess the correlation between mitotic count and Ki67, tumor grade and mitotic count as well as tumor grade and Ki67.
Results
There were 40 cases of NET diagnosed in the study period which included 22 male and 18 female patients in the age range of 24 to 80 years (mean: 60.1 years) Majority of them (33 cases, 82.5%) were >40 years of age. The diagnosis was made on endoscopic biopsies in 12 cases and resected surgical specimens were available in 28 patients. Biopsy diagnosis was mostly made in NETs of GI tract whereas all the pancreatic and periampullary lesions were diagnosed on resected specimens.
Clinical features
All the cases were non-functional and most common presentation was abdominal pain, loss of weight and loss of appetite. The pancreatic and periampullary lesions presented with obstructive jaundice for which Whipple’s resection was done. None of the cases presented with carcinoid syndrome or symptoms related to hormonal secretion. All were sporadic cases of NET and syndromic association with MEN was not identified. The clinical features are summarized in Table 1.
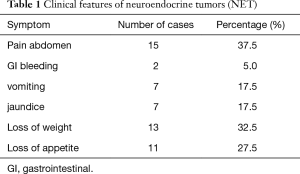
Full table
Location
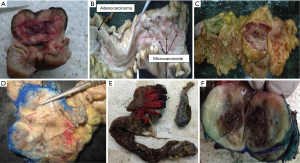
GI NETs were identified in 29 patients whereas 11 were pancreatic NETs. In GIT, the commonest location was duodenum and periampullary region (n=9, 22.5%) followed by stomach (7 cases, 17.5%) rectum (6 cases, 15%) ileum (4 cases, 10%) and colon (2 cases, 5%). Only a single case of jejunal NET was identified. Out of the 11 cases of pancreatic NET, 7 were arising from head of pancreas, 2 from the body of pancreas and 2 from tail of the pancreas. The gross photographs of the excised specimens are depicted in Figure 1.
Histopathology
The most frequent pattern of cellular arrangement was that of islands and lobules (23 cases, 57.5%).The cells showed characteristic monomorphic nuclei with stippled chromatin and granular eosinophilic cytoplasm. Trabeculae and sheets were identified infrequently (9 cases, 22.5% and 5 cases, 12.5% respectively) whereas true gland formation was the least frequent pattern (3 cases, 7.5%). Majority of cases from small intestine and pancreas had shown islands as predominant histologic pattern (7 cases, 17.5% and 6 cases, 15% respectively). Trabecular pattern was observed mostly in pancreas and rectum (4 cases, 10% and 3 cases, 7.5%). Glandular pattern was observed only in duodenum (3 cases, 7.5%).The histomorphological features of tumors in the resected specimens are summarized in Table 2. IHC with chromogranin was positive in 38 out of 40 cases (95%) and synaptophysin in 20 out of 24 cases (83.3%). NSE was done in four cases and all were positive whereas pancytokeratin was done in 13 cases of which 11 were positive.
Full table
Grading of the NETs
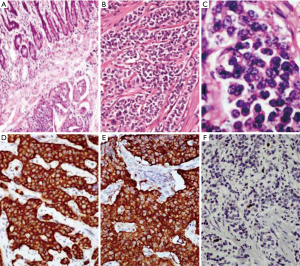
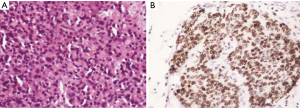
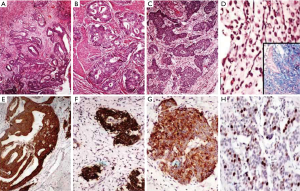
The grading according to the WHO criteria included 14 cases of grade 1, 9 cases of grade 2 and 5 cases of grade 3 tumors. The grading varied according to the location as summarized in Table 3. All the duodenal tumors were grade 1 (Figure 2) whereas 50% of colorectal tumors were grade 3 (Figure 3). The mean mitotic count and ki67 was in concordance with the grading of the tumors at various sites. The grading as well as the mean mitotic count and mean Ki67 are summarized in Table 3. There was a single case of gastric mixed adenoneuroendocrine carcinoma (MANEC). The adenocarcinoma was signet ring cell type whereas the NET was G2 (Figure 4).
Full table
Spearman’s correlation analysis
Spearman’s correlation coefficient is a statistical measure of the strength of a monotonic relationship between paired data. It is denoted by rs and is by −1< rs <1.
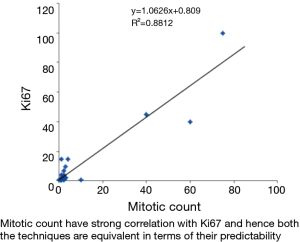
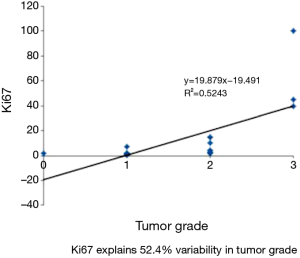
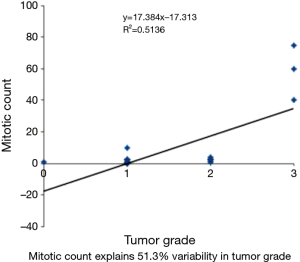
The variables mitotic count and Ki67 show very strong correlation using spearman correlation analysis with rs value of 0.88 (Figure 5). Whereas spearman correlation analysis with tumor grade and Ki67 show moderate correlation with rs value of 0.524 (Figure 6). Similarly correlation coefficient between mitotic count and tumor grade was 0.51 indicating moderate correlation between the two variables (Figure 7).
Discussion
The nomenclature and classification of NET has undergone significant change in last few years. The GEPNETs are rare tumors accounting for 2.5 to 5 cases per 100, 000 (4). The incidence of these tumors is on rise especially the gastric and the rectal tumors. However there are very few concise reports which give the entire spectrum and prevalence of these tumors in the GI tract and pancreas. In this study we attempt to put forward our experience of NET of GEP tract.
The increasing use of endoscopic study and rising trend of performing endoscopic biopsies from suspicious foci with easy availability of IHC stains perhaps have useful in documenting more and more number of GI NETs. In the present study surgical resection was performed in 28 patients and 8 of these were diagnosed on biopsies. Similar to our study, Maggard et al. and Modlin et al. observed that the average age for NETs at diagnosis were 60.9 and 61.4 years respectively (5,6).
All the cases diagnosed in the present study were sporadic and we did not identify any case in association with MEN syndrome which is a common syndromic association of these tumors. All the patients presented with nonspecific symptoms of abdominal pain and vomiting. Similar findings have been noted by Amarapurkar et al. who reported 74 cases of NETs of GIT-pancreas (2). Earlier ileum and appendix have been reported as the most common sites for NET (7). However in the present study the GI tumors were more frequently encountered in comparison to pancreatic NETs with commonest location being duodenum and periampullary region. Similar findings were also noted by Maggard et al. small intestine was the most common site accounting for 44.7% (5). This is in contrast to the study by Amarapurkar et al. where stomach (30.2%) was found to be the most common site followed by pancreas (23.3%) (2).
The gastric carcinoids are divided into three types and the most frequent subtype is type I arising in the fundus or body and these are associated with chronic atrophic gastritis. In our study most of the gastric carcinoids were type II (78.5%) followed by type I (28.5%). The third type of gastric NET comprises of sporadic tumors and do not show any evidence of atrophy or hyperplasia. These tumors unlike the other two subtypes are more often large with a higher grade, and have a worse prognosis (8).
The duodenal carcinoids are indolent tumors and natural history of these tumors is not well established. They are usually smaller than 2 cm and identified on endoscopic evaluation. Five of the 7 duodenal NETs were identified on endoscopic biopsies. The mean age (63.5 vs. 57 years), mean mitotic count (1/10 HPF), Ki67 (1%) and grade of the tumor (NET G1) were comparable with the study done by Ishido et al. (9). The clinical course of the ampullary NETs are different from duodenal NETs, as they have a more aggressive phenotype, with generally higher-grade tumor (10).
There was a single case of small bowel carcinoid presenting with 44 polyps in the duodenum. Multiplicity in small intestinal carcinoids is reported in 20–30% cases and the significance of multiplicity is not well established. These patients are seen to be younger and have more propensities for carcinoid syndrome. However in our study the patient was 68 years old and presented with obstructive symptoms for which Whipple’s resection was performed. These tumors are generally associated with synchronous adenocarcinomas and to have poor prognosis as compared to solitary tumors. Another case of total proctocolectomy specimen had adenocarcinoma and multiple carcinoids (grade 1) in the background of ulcerative colitis. MANECs of the upper GI tract are rare, and in the small intestine these tumors are most commonly located in the ampullary region. These constitute a morphologically distinct population (30%) of mucin-producing adenocarcinoma or rarely squamous cell carcinoma, intimately admixed intermixed or adjacent to a neuroendocrine component. The neuroendocrine component is generally low grade and is rarely high grade (11). MANECs should be considered as carcinomas. Grassia et al. almost reported two similar cases in colorectal region with ki67 50% and 90% and thus were labelled as grade 3 tumors (12).
Colorectal carcinoids account for 4–8% of the GIT NETs. The incidence was slightly higher in our study (8 cases, 27.5%) These are regarded as “low-grade malignant”, even in the presence of metastasis. Furthermore, the WHO classification defines colorectal carcinoids as “benign” if the tumors are localized in the sub mucosa, measuring 20 mm or less and lack vascular invasion (3).
NET exhibit varied morphologic patterns. However the well differentiated NETs show characteristic cellular arrangement in the form of islands and lobules. The neuroendocrine nature of cells can be identified by stippled nuclear chromatin and granular cytoplasm. Poorly differentiated tumors however require help of IHC for the diagnosis (7). According to Joseph et al. and Simpson et al. NSE is diffusely expressed in the cytoplasm of all the GEPNETs (13,14). In our study NSE was done in four cases and was positive in all the cases.
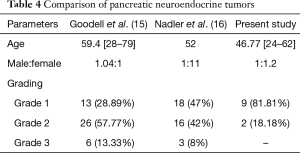
A comparative analysis of the pancreatic NETs is summarized in Table 4. Grading of the NETs is a very important issue and one of the most important prognostic parameters. The proliferative rate provides significant prognostic information for NETs (7). Use of mitotic count for exact grading is problematic since it shows subjective variations. Moreover in biopsy exact mitosis cannot be documented because of limited tissue. The proliferation markers like Ki67 index have thus emerged as an important basis for grading. The proliferation rates are reported as percentage positivity after assessing the positive nuclei in 100 cells. This has been shown to have good interpersonal and inter institutional reproducibility in a study by Nadler et al. (16).
Full table
Yamaguchi et al. reported metastatic deposits in 7 out of the 45 cases whereas in our study 3 out of 40 cases showed metastasis to distant lymph nodes and one case to liver (17). We found that majority of the tumors were grade 1 (G1). However the grading varied as per the location of the tumor. The colorectal tumors were found to be more poorly differentiated (G3) in comparison to the tumors at other locations in GIT and pancreas. The same has been reported in literature. In general, the well-differentiated NETs are much more common than the poorly differentiated counterparts. However, at certain locations such as the esophagus or colon the poorly differentiated NETs were more frequently encountered. Estrozi B noted stomach followed by small intestine and pancreas as common sites with majority being G1 tumors (18). In the present study majority of tumors in pancreas were grade 1 (81.81%) and the rest grade 2 (18.18%). Goodell et al. found that 57.77% of the cases belonging to grade 2 followed by G1 and G3. There was a strong correlation between mitotic count and ki67 labelling index (15). In a study done by Kımıloğlu Şahan et al. on 21 cases of GEPNET showed a significant correlation with spearman correlation analysis with r=0.684 whereas our study has shown a moderate correlation with r=0.524 (19).
Apart from grading and location, staging of the primary tumor is an important prognostic parameter in NETs. These tumors also are staged according to the TNM staging system. Of the 28, resected specimens, it was found that 39% were already stage T3 with infiltration into subserosa. The localized disease was found in 43% of tumors and 57% had regional lymph node metastasis. However distant metastasis was seen in only one case. The stage of the tumor and the location did not show any correlation. The tumors with metastasis are referred to as neuroendocrine carcinomas irrespective of the grade of the tumor. Levi et al. in their report have used the basis of local infiltration and/or existence of metastasis to classify benign and malignant NETs (20). But the concept of “benign” NET is not followed anymore and all NETs are treated as potentially malignant tumors. A single case of MANEC of stomach was identified. According to 2010 WHO classification system the tumors that show an additional non-endocrine component (usually adenocarcinoma) comprising of at least 30% of all tumor cells are called as MANEC (3). Literature describes very early reports of tumors showing mixed adenocarcinoma component with focal neuroendocrine features. However these tumors were previously sub classified as collision tumors, combined tumors, and exocrine tumors. Both the components of the tumor show features of malignancy. These are very rare tumors and the adenocarcinoma component can show features of any morphologic type. In gastric MANEC; however the adenocarcinoma component is frequently signet ring cell type similar to that seen in our patient. The prognosis depends on the stage and metastasis. The present tumor was a T3 tumor with extensive perineural and perivascular invasion with lymph node metastasis. Park et al. evaluated presence of perineural invasion and lymphovascular emboli as one of the prognostic variables (21).
Conclusions
The small intestines followed by pancreas were found to be the most common sites of NET of GI tract and pancreas. Majority of the tumors are NET G1 and were classified based on WHO classification. Tumors from colorectal region were mostly NEC G3. This study had a significant correlation with respect to Ki67 LI and the mitotic count and moderate correlation with respect to Ki67 and tumor grade. Thus Ki67 was helpful in grading these tumors and elucidation of these features will facilitate early diagnosis and improve the accuracy of grading of tumors to predict the therapy and outcome of the disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Warner RR. Enteroendocrine tumors other than carcinoid: a review of clinically significant advances. Gastroenterology 2005;128:1668-84. [Crossref] [PubMed]
- Amarapurkar DN, Juneja MP, Patel ND, et al. A retrospective clinico-pathological analysis of neuroendocrine tumors of the gastrointestinal tract. Trop Gastroenterol 2010;31:101-4. [PubMed]
- Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, et al. editors. WHO classification of tumors of the digestive system. Lyon: International Agency for Research on Cancer (IARC), 2010:13-4.
- Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61-72. [Crossref] [PubMed]
- Maggard MA, O'Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg 2004;240:117-22. [Crossref] [PubMed]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [Crossref] [PubMed]
- Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707-12. [Crossref] [PubMed]
- Grin A, Streutker CJ. Neuroendocrine tumors of the luminal gastrointestinal tract. Arch Pathol Lab Med 2015;139:750-6. [Crossref] [PubMed]
- Ishido K, Tanabe S, Higuchi K, et al. Clinicopathological evaluation of duodenal well-differentiated endocrine tumors. World J Gastroenterol 2010;16:4583-8. [Crossref] [PubMed]
- Randle RW, Ahmed S, Newman NA, et al. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: a population-based study. J Gastrointest Surg 2014;18:354-62. [Crossref] [PubMed]
- Vilardell F, Velasco A, Cuevas D, et al. Composite papillary intestinal-type adenocarcinoma/poorly differentiated neuroendocrine carcinoma of the ampulla of Vater. J Clin Pathol 2011;64:174-7. [Crossref] [PubMed]
- Grassia R, Bodini P, Dizioli P, et al. Neuroendocrine carcinomas arising in ulcerative colitis: coincidences or possible correlations? World J Gastroenterol 2009;15:4193-5. [Crossref] [PubMed]
- Joseph T, Shanthala PR. Gastroenteropancreatic Neuroendocrine Tumours – An Institutional Experience. Int J Biomed Res 2015;6:71-6. [Crossref]
- Simpson S, Vinik AI, Marangos PJ, et al. Immunohistochemical localization of neuron-specific enolase in gastroenteropancreatic neuroendocrine tumors. Correlation with tissue and serum levels of neuron-specific enolase. Cancer 1984;54:1364-9. [PubMed]
- Goodell PP, Krasinskas AM, Davison JM, et al. Comparison of methods for proliferative index analysis for grading pancreatic well-differentiated neuroendocrine tumors. Am J Clin Pathol 2012;137:576-82. [Crossref] [PubMed]
- Nadler A, Cukier M, Rowsell C, et al. Ki-67 is a reliable pathological grading marker for neuroendocrine tumors. Virchows Arch 2013;462:501-5. [Crossref] [PubMed]
- Yamaguchi T, Fujimori T, Tomita S, et al. Clinical validation of the gastrointestinal NET grading system: Ki67 index criteria of the WHO 2010 classification is appropriate to predict metastasis or recurrence. Diagn Pathol 2013;8:65. [Crossref] [PubMed]
- Estrozi B, Bacchi CE. Neuroendocrine tumors involving the gastroenteropancreatic tract: a clinicopathological evaluation of 773 cases. Clinics (Sao Paulo) 2011;66:1671-5. [PubMed]
- Kımıloğlu Şahan E, Erdoğan N, Ulusoy İ, et al. P53, KI-67, CD117 expression in gastrointestinal and pancreatic neuroendocrine tumours and evaluation of their correlation with clinicopathological and prognostic parameters. Turk J Gastroenterol 2015;26:104-11. [Crossref] [PubMed]
- Levi F, Te VC, Randimbison L, et al. Epidemiology of carcinoid neoplasms in Vaud, Switzerland, 1974-97. Br J Cancer 2000;83:952-5. [Crossref] [PubMed]
- Park JY, Ryu MH, Park YS, et al. Prognostic significance of neuroendocrine components in gastric carcinomas. Eur J Cancer 2014;50:2802-9. [Crossref] [PubMed]