Acute pancreatitis as a complication of trans-arterial chemoembolization of hepatocellular cancer—case report and review of literature
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in the world and the leading cause of cancer-related deaths worldwide (1). Prognosis is typically poor because many patients are burdened with unresectable tumors (2). Well differentiated HCC is mostly dependent on the hepatic artery for blood supply and it is through this characteristic that transarterial chemoembolization (TACE) procedure is used as an effective palliative treatment for HCC. The procedure is performed by injecting drug eluting beads loaded with chemotherapy agent into the vasculature supplying the HCC which serves a dual purpose of blocking the blood supply and targeting delivery of the chemotherapeutic agent to the tumor. Complications of TACE can result from inadvertent embolization of arteries supplying the liver, gallbladder, stomach and pancreas. Here we present a patient with HCC who developed a rare complication of acute pancreatitis following TACE procedure, likely owing to his variant arterial anatomy.
Case presentation
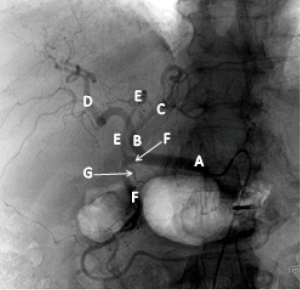
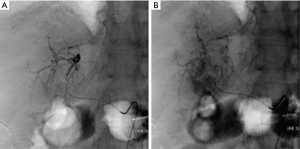
A 65-year-old Caucasian male with history of chronic hepatitis C and cirrhosis was diagnosed with multifocal HCC with a dominant lesion in the medial left lobe of the liver. TACE was performed via the right trans-femoral approach. Aortogram, superior mesenteric arteriogram and celiac artery (CA) injections were performed. Celiac arteriogram and subsequent selective arteriogram of the common hepatic artery (CHA) showed variant arterial anatomy with the middle hepatic artery (MHA) arising directly from the gastroduodenal artery (GDA) in close proximity to the origin of the superior pancreatic artery (Figure 1). Super selective catheterization of the MHA (supplying the medial segment of left hepatic lobe) was performed and this was found to be supplying the suspected lesion (Figure 2). Embolization was carried using 70–150 microns microsphere beads (LC Bead M1, BTG, West Conshohocken, PA,USA) loaded with 50 mg of doxorubicin and lipiodol. Subsequently, the lateral segment of left hepatic artery and the right hepatic artery were also embolized using 100–300 microns beads loaded with 50 mg of doxorubicin along with 3 mL of lipidil (Guerbet, Bloomington, IN, USA). The patient was administered prophylactic antibiotics including cefazolin and metronidazole in the immediate pre and post procedure period per protocol.
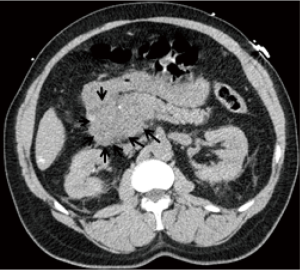
Patient developed significant epigastric pain within 24 hours of TACE procedure. His CBC showed an elevated WBC count of 15.4 t/c mm. Serum amylase and lipase levels were 400 (ref: 28–100) and 3,809 (ref: 73–383) U/L respectively. His liver function tests showed moderate elevation of serum AST and ALT at 314 (ref: 9–14) and 168 (ref: 17–63) IU/L respectively. Serum alkaline phosphatase and total bilirubin were normal. His right upper quadrant ultrasound did not show gallstones or biliary dilation. Computer tomography scan of the abdomen performed without contrast showed new finding of enlarged head of the pancreas with peri-pancreatic fat stranding consistent with acute pancreatitis (Figure 3). A diagnosis of TACE induced acute pancreatitis was made and patient was treated conservatively. His course of hospitalization was uneventful and he was discharged 72 hours later, in a stable condition. He appeared well 4 weeks after discharge, without further complications. He was subsequently treated again 5 months later to the same artery with the same reagents but utilizing a special micro catheter designed to act as a one way valve during bead delivery to prevent reflux (Surefire Medical, Westminster, CO, USA). The patient tolerated this procedure well and was discharged the next day without any signs of complication.
Discussion
TACE is a common procedure used to treat patients with non-resectable HCC. The therapeutic effect is based on the differential arterial supply of HCC by the hepatic artery while the rest of the liver parenchyma is usually supplied >70% by the portal vein. TACE is used to super-selectively block the arterial supply of the tumor without deleterious effect on rest of the liver. Drug eluting beads coated with doxorubicin is the most common chemotherapeutic agent used for chemo-embolization.
It is imperative for interventional radiologists involved in performing TACE be aware of the anatomical variation of the hepatic arterial circulation to prevent inadvertent embolization and complications. The intra-abdominal portion of aorta most commonly gives rise to three main anteriorly directed branches before its bifurcation. They include the CA, superior mesenteric artery (SMA) and the inferior mesenteric artery (IMA). CHA arises from CA. CHA gives rise to hepatic artery proper (HAP), right gastric artery (RGA) and GDA. Recently, Sureka et al. conducted a retrospective study of 600 subjects to study the variations in hepatic arterial circulation using multidetector CT (MDCT) scan (3). The left hepatic artery (LHA) and right hepatic artery (RHA) originated from HAP in 80% of the cases. The MHA originated from LHA or RHA in 69% of cases. Unusual variations included origin of CHA from SMA or aorta, origin of RHA from SMA, CA or aorta and origin of LHA from LGA or aorta. MHA rarely originated from CHA or an accessory hepatic artery. GDA originated from CHA in 98% while directly originating from the CA, RHA or LHA in less than 2% of cases.
Acute pancreatitis is a rare complication of TACE. The proposed mechanism involves regurgitation of the embolic beads from the hepatic artery into an artery supplying the pancreas, especially the GDA (4), resulting in ischemia of the pancreas. It is important to note that the head of the pancreas is particularly susceptible to ischemic injury since the arteries supplying it (pancreaticoduodenal arcades anterior and posterior) are terminal arteries. The rest of the pancreas, which is richly supplied by branches of SMA, is rarely the primary site of injury (4). The incidence of acute pancreatitis following TACE ranges from 1.7% to 15.2% (4,5). Acute pancreatitis can develop within 24 hours to up to 15 days after TACE procedure (6,7). The severity can range from mild (4) to necrotizing pancreatitis (6-12). In the largest study by López-Benítez et al., evaluating risk factors for acute pancreatitis following liver embolization (bland or chemotherapeutic), volume of embolic microspheres and use of carboplatin were most significantly associated with TACE induced pancreatitis (4). Carboplatin, when given systemically has been associated with acute pancreatitis (13). Anatomical variations of hepatic artery or arterio-venous fistulae, size of microspheres or use of lipiodol, were not associated with post TACE acute pancreatitis.
Although prior authors have stated anatomic variation is not necessarily associated with pancreatitis following TACE, it is likely that specific variation presented here is so rare that it would be difficult to have a study that was powered to truly detect whether anatomic variations had a significant impact on pancreatitis post-TACE. It stands to reason that larger volumes administered lead to increased stasis in the target artery and thus increased likelihood of reflux of embolic material with resultant non-target embolization. Non-target embolization of pancreatic arterial supply is the likely culprit for pancreatitis, regardless of anatomy. In our case, a reasonable conjecture is that the close proximity of the superior pancreaticoduodenal artery to the target artery makes even a small amount of reflux a risky proposition. As with every case, embolization was carried out under direct visualization watching for stasis in of flow in the artery and stopping before any reflux was identified. It seems likely, though, that a small amount of material refluxed undetected. Even a small amount of reflux when combined with the anatomic variant could have contributed to the development of pancreatitis.
The most common adverse effect of TACE is post-embolization syndrome and occurs in 60–80% of patients (14). This comprises symptoms of abdominal pain, fever and ileus as a result of local cytotoxicity and tumor ischemia. Other immediate complications include chemical cholecystitis (5), and tumor rupture (15). As most of these complications present with post procedure abdominal pain, laboratory investigations and further imaging is often necessary to determine the etiology of the abdominal pain. Long term complications of TACE include gastric ulcer (14), liver abscess (16) and biliary strictures (17). Rarely, arterio-venous shunts result in systemic complications including pulmonary embolization (18) and contrast induced acute renal failure (19). In a study by Xia et al., the total incidence of a severe complication from TACE of the liver was 2.68% (18). Obtaining a good arteriogram and identification of anatomical variations and arterio-venous shunts combined with targeted chemo-embolization may avoid most of TACE induced complications.
Conclusions
We report a case of acute pancreatitis that developed as a complication of TACE procedure that was performed for HCC. Even though, a super selective catheterization of the MHA supplying the tumor in the medial left lobe was achieved, the anatomical variation of this artery arising directly from the GDA, instead of from the proper hepatic artery, may have resulted in reflux of the embolic beads into the GDA and acute pancreatitis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Bosch FX, Ribes J, Cléries R, et al. Epidemiology of hepatocellular carcinoma. Clin Liver Dis 2005;9:191-211. v. [Crossref] [PubMed]
- Greten TF, Papendorf F, Bleck JS, et al. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer 2005;92:1862-8. [Crossref] [PubMed]
- Sureka B, Mittal MK, Mittal A, et al. Variations of celiac axis, common hepatic artery and its branches in 600 patients. Indian J Radiol Imaging 2013;23:223-33. [Crossref] [PubMed]
- López-Benítez R, Radeleff BA, Barragán-Campos HM, et al. Acute pancreatitis after embolization of liver tumors: frequency and associated risk factors. Pancreatology 2007;7:53-62. [Crossref] [PubMed]
- Stefanini GF, Amorati P, Biselli M, et al. Efficacy of transarterial targeted treatments on survival of patients with hepatocellular carcinoma. An Italian experience. Cancer 1995;75:2427-34. [Crossref] [PubMed]
- Komekado H, Kokuryu H, Kimura T, et al. Two cases of acute necrotizing pancreatitis complicating after transcatheter arterial embolization for hepatocellular carcinoma. J Gastroenterol 2005;40:107-8. [Crossref] [PubMed]
- Ozçinar B, Güven K, Poyanli A, et al. Necrotizing pancreatitis after transcatheter arterial chemoembolization for hepatocellular carcinoma. Diagn Interv Radiol 2009;15:36-8. [PubMed]
- Alcívar-Vásquez JM, Ontanilla-Clavijo G, Ferrer-Ríos MT, et al. Acute necrotizing pancreatitis after transarterial chemoembolization of hepatocellular carcinoma: An unusual complication. Rev Esp Enferm Dig 2014;106:147-9. [Crossref] [PubMed]
- Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol 2012;18:321-5. [Crossref] [PubMed]
- Chey V, Chopin-laly X, Micol C, et al. Acute pancreatitis after transcatheter arterial chemoembolization for liver metastases of carcinoid tumors. Clin Res Hepatol Gastroenterol 2011;35:583-5. [Crossref] [PubMed]
- Green TJ, Gipson MG. Acute pancreatitis after transarterial chemoembolization of hepatocellular carcinoma with drug-eluting beads. Semin Intervent Radiol 2015;32:18-21. [Crossref] [PubMed]
- Rodríguez-Grau MC, Jusué V, Fiera A, et al. Acute pancreatitis as fatal complication after chemoembolization of hepatocellular carcinoma. Rev Esp Enferm Dig 2014;106:146-7. [Crossref] [PubMed]
- Singh V, Devata S, Cheng YC. Carboplatin and docetaxel-induced acute pancreatitis: brief report. Int J Clin Oncol 2010;15:642-4. [Crossref] [PubMed]
- Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol 2009;10:425-34. [Crossref] [PubMed]
- Jia Z, Tian F, Jiang G. Ruptured hepatic carcinoma after transcatheter arterial chemoembolization. Curr Ther Res Clin Exp 2013;74:41-3. [Crossref] [PubMed]
- Kim W, Clark TW, Baum RA, et al. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol 2001;12:965-8. [Crossref] [PubMed]
- Kim HK, Chung YH, Song BC, et al. Ischemic bile duct injury as a serious complication after transarterial chemoembolization in patients with hepatocellular carcinoma. J Clin Gastroenterol 2001;32:423-7. [Crossref] [PubMed]
- Xia J, Ren Z, Ye S, et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol 2006;59:407-12. [Crossref] [PubMed]
- Huo TI, Wu JC, Lee PC, et al. Incidence and risk factors for acute renal failure in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: a prospective study. Liver Int 2004;24:210-5. [Crossref] [PubMed]


