Biological agents in gastrointestinal cancers: adverse effects and their management
Introduction
Gastrointestinal (GI) cancers constitute a wide spectrum of malignancies with wide variations in incidence, histopathological features, molecular characteristics and prognosis. Colorectal cancer is the 4th most common cancer in the United States, and the most common GI cancer. An estimated 134,490 new cases and 49,190 deaths attributable to CRC are expected in 2016. Despite major advances in the understanding of pathogenesis, epidemiology and treatment options, the 5-year survival stands at only 65.1%. The survival rates for other GI malignancies stand at similar or even lower rates—anal cancer (66.4%), gastric cancer (30.1%), esophageal cancer (18.4%) or pancreatic cancer (7.7%) (1).
Biologic therapy involves the use of living organisms, substances derived from living organisms, or laboratory-produced versions of such substances to treat disease. Monoclonal antibodies are laboratory produced antibodies that bind to certain antigens on cancer cells. Over the past 30 years, the Food and Drug Administration (FDA) has approved biologics for a wide variety of conditions. In the setting of malignancy, they can kill cancer cells by inciting an immune response to the cancer (rituximab), inhibiting signals that suppress the patient’s own immune response to the cancer (ipilimumab, pembrolizumab), affecting the tumor microenvironment or interfering with action of proteins or factors necessary for cancer growth [e.g., inhibition of vascular endothelial growth factor (anti-VEGF), inhibition of epidermal growth factor receptor (anti-EGFR)] (2,3). Monoclonal antibody therapy for GI cancers, though a recent advancement, is currently a well-established addition to chemotherapy to improve response rates and overall survival.
Multiple biologic therapies are approved for use in patients with GI cancers including agents targeting VEGF [bevacizumab, aflibercept in metastatic colorectal cancer (mCRC) and ramucirumab in metastatic colorectal and gastric cancer], agents targeting EGFR (cetuximab and panitumumab in mCRC without RAS mutations) and one agent targeting human epidermal growth factor receptor 2 (Her2/Neu) (trastuzumab in HER2 amplified, metastatic gastro-esophageal adenocarcinoma). In addition, several immunotherapy drugs are under intensive evaluation for GI cancer therapy in multiple early phase clinical trials (pembrolizumab, nivolumab, atezolizumab, durvalumab), and appear to be active in patients with microsatellite unstable tumors. These agents have adverse events that are distinct from the chemotherapeutic agents they are often combined with, depending on their target signalling pathway. This review aims to characterize the adverse events associated with use of biologic agents in GI malignancies, and summarize best practices for managing these adverse events.
Anti-angiogenic agents
Angiogenesis plays a critical role in the growth and spread of cancer, and requires the binding of signaling molecules, such as VEGF, to receptors on the surface of normal endothelial cells. Angiogenesis inhibitors interfere with various steps in this process. Bevacizumab is a recombinant humanized monoclonal antibody that targets VEGF-A, and was approved by the FDA in 2004 to treat mCRC as a combination with fluorouracil based regimens. Aflibercept was FDA approved in 2012 for use in combination with chemotherapy for mCRC. It is a recombinant, decoy receptor fusion protein, designed to target VEGF-A, VEGF-B and placental growth factor (4). Ramucirumab is a recombinant, monoclonal immunoglobulin G1 antibody that binds VEGFR-2 and blocks the binding of VEGF-A, VEGF-C and VEGF-D (5). It was approved in 2014 for the treatment of advanced gastric or gastroesophageal junction carcinoma, either alone or in combination with paclitaxel, and subsequently in 2015 for second line therapy of mCRC patients, in combination with fluorouracil and irinotecan.
Adverse events often seen in association with use of anti-angiogenic agents are hypertension, proteinuria, thromboembolism, hemorrhage, delayed wound healing and increased wound complications and GI perforation.
Hypertension
Hypertension is a frequent adverse event attributable to the anti-angiogenic effects of VEGF inhibitors. Grade 3–4 hypertension is reported with a frequency of up to 17.4% in clinical trials evaluating combination of chemotherapy and anti-angiogenic agents (6-8). The development of hypertension is hypothesized to be due to reduced nitric oxide production and rarefaction of vessels (9,10). Hypertension in response to bevacizumab may also have a genetic component (11,12).
Hypertension can develop at any time during treatment, and can be dose related. All patients who are beginning therapy with angiogenesis inhibitors should have a formal cardiovascular risk assessment, including blood pressure (BP) monitoring at start of therapy and every 2–3 weeks thereafter as long as BP is stable (13). It is important to note that pre-existing hypertension is common in cancer patients (14), and this can worsen while on VEGF inhibitor therapy (15).
The goal of management is to keep the BP below 140/90 mmHg, and, in certain populations like diabetes mellitus or chronic kidney disease patients, below 130/80 mmHg. Drug therapy for VEGF inhibition induced hypertension includes usual anti-hypertensive agents. VEGF inhibition induced rise in BP dissipates after cessation of the drug. It is prudent to anticipate a fall in the BP upon cessation of VEGF inhibitor therapy and adjust the antihypertensive medications accordingly (16). VEGF inhibitors should not be initiated in patients with uncontrolled hypertension. Permanent discontinuation of therapy may be necessary if systolic BP is >200 mmHg or diastolic BP is >100 mmHg, hypertension is unmanageable with oral antihypertensive agents or in the event of hypertensive crisis (17,18).
Proteinuria
The incidence of all grade proteinuria attributable to angiogenesis inhibition is up to 63%, while the incidence of grade 3–4 proteinuria has been reported to be up to 7% (19). Proteinuria has been correlated with presence of hypertension and the dose of the VEGF inhibitor. All patients should be screened for proteinuria before initiation of therapy, along with BP monitoring and estimation of renal function. If there is no evidence of proteinuria, patients should have repeat screening before each cycle. If screening reveals grade 1+ proteinuria, then urinary protein excretion should be quantified using a spot urine protein/creatinine ratio or a 24-hour urine protein measurement (13).
Therapy should be discontinued for proteinuria >2 g/24 h or spot urine protein/creatinine ratio >2, until it returns to baseline (18). ACE inhibitors are a therapeutic option to combat proteinuria in addition to controlling rise in BP. A kidney biopsy may be necessary in cases of progressive renal disease, unexplained renal failure or nephritic syndrome to exclude other etiologies (13). Onset or relapse of minimal change disease in the setting of bevacizumab therapy has also been described (20,21).
Thromboembolic events
Addition of bevacizumab to standard chemotherapeutic options for GI malignancies increases the risk of arterial thrombotic events (ATE) but not that of venous thromboembolic events (22-24). An ATE can manifest as myocardial infarction, cerebrovascular accident, sudden cardiac death and transient ischemic attack (25).
This increased risk is likely related to the loss of and nitric oxide and prostacyclin production, which normally inhibit platelet aggregation. Inhibition of VEGF could cause defects in the endothelium that expose pro-coagulant phospholipids on the luminal membrane leading to thrombosis or hemorrhage (26). ATE have been reported to be more frequent in those with proteinuria. These drugs should be discontinued in anyone who experiences a severe ATE, while on therapy. These patients can receive full dose anticoagulation without any increased risk of grade ≥3 bleeding (18).
Though the current evidence to use low dose aspirin for prophylaxis of ATEs in patients receiving bevacizumab is limited, the concomitant use of bevacizumab, chemotherapy and aspirin does not appear to increase bleeding risk compared to chemotherapy plus aspirin alone. The decision to use aspirin for prophylaxis in patients receiving anti-angiogenic agents needs to be individualized based on risk factors and lack of contraindications to aspirin use (18,23).
Bleeding events
Anti-angiogenic agents can cause two distinct forms of bleeding—minor hemorrhage which is most commonly epistaxis, and major bleeding events including but not limited to GI, central nervous system, and vaginal bleeding, and hemoptysis.
Low grade mucocutaneous bleeding such as epistaxis does not usually require specific treatment and does not require treatment discontinuation.
A meta-analysis of nine studies utilizing bevacizumab for the treatment of colorectal cancer reported an overall incidence of grade 3–4 bleeding events of 1.8% (8). This was similar to the incidence reported by the Bevacizumab Regimens’ Investigation of Treatment Effects (BRiTE) study (2.2%; 95% CI, 1.6–2.9%). The majority of the events in the BRiTE study were GI or rectal bleeds.
To minimize the risk of severe bleeding in the setting of bevacizumab therapy, it is imperative that patients be evaluated for potential risk factors for bleeding (27). Bevacizumab should not be administered to patients with serious hemorrhage or recent hemoptysis and should be discontinued upon development of any serious bleeding event. Similarly, aflibercept should be avoided in patients with a bleeding diathesis or that receiving full dose anticoagulation (28).
GI perforation
In a meta-analysis of 33 randomized controlled trials utilizing bevacizumab, the incidence of GI perforation was reported to be 1.1% (95% CI, 0.8–1.5%) with an overall incidence of bevacizumab-associated GI perforation related mortality (grade 5) of 8.8% (95% CI, 5.3–14.3%) (29). Both low and high doses of bevacizumab are associated with increased risk of GI perforation, and the risk has been reported to be dose dependant (30). A similar rate of GI perforation has been reported with aflibercept (1.9%, 95% CI, 1.0–3.8%) with a mortality of 10.8% (95% CI, 4.1–25.5%) (31).
Possible mechanisms of GI perforation include limitation of blood flow to the GI tract leading to bowel infarction and perforations (30).
Patients at higher risk for perforation should be identified prior to initiation of therapy, such as those with history of diverticulitis or peptic ulcer disease, radiation exposure, obstruction, recent endoscopy and multiple previous surgeries. If perforation is detected, prompt surgical assessment is necessary along with bowel rest, fluid resuscitation and intravenous broad spectrum antibiotics. A single centre study of patients who developed perforation while on bevacizumab revealed that the majority (79%) of patients were successfully managed non-operatively (32). However these decisions need to be individualized based on severity and clinical presentation.
Postoperative wound healing complications
The BRiTE study reported a 4.4% (95% CI, 2.7–6.2%) incidence of postoperative wound healing complications in patients who underwent a surgical procedure within 90 days of the last dose of bevacizumab (25). Among the listed complications were wound dehiscence, wound bleeding and wound infections.
VEGF is involved in three physiological responses to tissue injury necessary for wound healing—vasodilation, increased vascular permeability and angiogenesis. Blocking of these essential responses by VEGF inhibitors is believed to be the cause of delayed wound healing and predisposition to complications for patients on these agents (33-35).
It is currently recommended that bevacizumab, ramucirumab and aflibercept be discontinued at least 4–6 weeks prior to elective surgery and therapy should not be resumed for at least 4 weeks after major surgery, until the surgical wound is completely healed (36).
Neutropenia and thrombocytopenia
Aflibercept in combination with folinic acid, fluorouracil, irinotecan (FOLFIRI) has displayed a higher incidence of neutropenic complications (febrile neutropenia and neutropenic infections) than FOLFIRI plus placebo (37,38). In the ramucirumab versus placebo in combination with second-line FOLFIRI in patients with mCRC that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE) trial, ramucirumab in combination with FOLFIRI had a 38% incidence of grade 3–4 neutropenia compared to 24% in FOLFIRI/placebo (39). The ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW) trial also reported higher incidence of neutropenia (41% vs. 19%) and leukopenia (17% vs. 7%) in ramucirumab treated groups compared to controls (40).
All patients should have a baseline complete blood count and differential prior to initiation of therapy as well as prior to each cycle. If neutrophil count falls below 1.5×109/L, therapy should be delayed until recovery to above 1.5×109/L (28).
EGFR inhibitors
The EGFR inhibitors cetuximab and panitumumab are approved for use in patients with RAS wild type mCRC. Cetuximab is a recombinant, human/mouse chimeric monoclonal antibody that binds to the extracellular domain of human EGFR, competitively blocks the binding of EGF and inhibits downstream signal transduction (41). Panitumumab is a fully human IgG2 anti-EGFR monoclonal antibody (42,43). The major toxicities reported for these agents are mucocutaneous, diarrhea, hypomagnesemia, and infusion reactions.
Mucocutaneous toxicity
One of the major adverse events of therapy with an anti EGFR agent is skin toxicity, which can manifests in several forms. The incidence of any grade skin toxicity during therapy with cetuximab or panitumumab ranges from 80–95%, and grade 3–4 toxicities ranges from 6–10% (44-49). Papulopustular rash and xerosis have even been studied as prognostic markers of response to therapy in patients treated with cetuximab or panitumumab (50). Skin rash is mostly mild-to-moderate in severity and requires therapeutic intervention in about one third of patients. Although the skin rash is self-limiting and usually resolves without scarring upon discontinuation of anti-EGFR therapy, the condition can negatively affect treatment compliance and quality of life (QOL). In addition to leaving skin vulnerable to superinfection, skin rash can lead to dose modification or treatment discontinuation, thus potentially affecting the overall clinical benefits of this form of therapy.
The basal layer of the epidermis has strong expression of EGFR which contributes to epidermal growth, wound healing and inhibition of differentiation. Inhibition of EGFR leads to impaired growth and migration of keratinocytes as well as inflammatory chemokine expression by these cells. This leads to inflammatory cell recruitment and cutaneous injury, resulting in toxicities seen with cetuximab or panitumumab (51).
Prophylactic measures have been evaluated as means to decrease the incidence or severity of skin reactions in response to anti-EGFR therapy. The skin toxicity evaluation protocol with panitumumab (STEPP) was the first prospective trial designed specifically to compare pre-emptive with reactive treatment for EGFR-inhibitor mediated skin toxicity. Patients receiving panitumumab in addition to FOLFIRI were randomly assigned to receive either pre-emptive treatment (daily skin moisturizer, sun-screen, 1% hydrocortisone cream, and doxycycline 100 mg twice daily, from 24 hours before their first dose of panitumumab through week 6) versus reactive treatment, after development of skin toxicity. The study revealed a significantly lower (29% vs. 62%) incidence of ≥ grade 2 skin toxicities during the 6-week period of therapy, coupled with lower rates of QOL impairment in the pre-emptive treatment group (52). A meta-analysis of 13 studies using anti-EGFR therapy for solid tumors revealed a 26% absolute difference in incidence of high grade acneiform skin rash when prophylactic antibiotics (tetracyclines) were used for several weeks prior to start of the anti EGFR therapy (53). Table 1 outlines the major mucocutaneous toxicities associated with cetuximab or panitumumab use, their reported incidence rates and optimal management strategies.
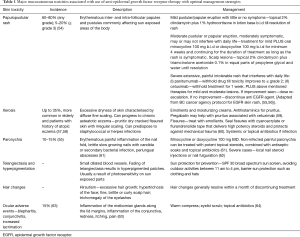
Full table
Hypomagnesemia
Hypomagnesemia is a common side effect of therapy with anti-EGFR agents. A recent systematic review reported the incidence of cetuximab related hypomagnesemia to be 35–100% for all grade and 1.7–27% for grade 3–4 (65). The incidence of grade 3–4 hypomagnesemia with panitumumab has been reported to be 4%, with an all grade incidence of 28.9–85.7% (65,66). Incidence of hypomagnesemia appears to be related to the duration of treatment. In a Belgian study with 98 mCRC patients treated with anti-EGFR therapy, 97% experienced a progressive decline in magnesium levels with a median time to onset of hypomagnesemia of 99 days (range, 12–639 days) (67). The incidence has been reported as 5% within 3 months, 23% within 3–6 months and 47% with greater than 6 months of treatment with cetuximab (68).
Hypomagnesemia can lead to cardiovascular (arrhythmias, hypertension, cardiomyopathy), neuromuscular (weakness, confusion, tetany, agitation, tremors) or behavioural (depression, delirium, psychosis) complications (69). Hypocalcemia can be associated as a result of hypomagnesemia induced parathyroid hormone resistance.
Hypomagnesemia should be suspected in patients who develop chronic diarrhea, arrhythmias, refractory hypokalemia or hypocalcemia during therapy with cetuximab or panitumumab (69). Electrolytes should be monitored periodically for 8 weeks after completion of anti-EGFR therapy. Table 2 outlines the management of hypomagnesemia.

Full table
Diarrhea
In EGFR monotherapy trials, the incidence of grade 3–4 diarrhea has been 1–2%. This incidence increased to 28% in trials combining cetuximab with chemotherapy (44,72-75). A 2015 meta-analysis of 18 studies and 13,382 patients revealed a 66% increased risk of developing grade 3–4 diarrhea while on treatment with cetuximab or panitumumab in combination with chemotherapy compared to chemotherapy alone (RR, 1.66; 95% CI, 1.52–1.80) (76). The reported overall incidence of grade 3–4 diarrhea was 18%, compared to 11% in the control arm. The same meta-analysis also reported a significantly higher risk of mucositis in patients receiving panitumumab or cetuximab as part of their therapy (RR, 3.44; 95% CI, 2.66–4.44). The incidence of severe mucositis was 8% in the experimental arm and 2% in the control arm.
Patients should be provided education for symptoms of severe diarrhea, dehydration and electrolyte disturbances at the start of treatment. Management of diarrhea includes bowel rest, hydration, electrolyte repletion, and anti-motility agents such as loperamide and diphenoxylate once infection is ruled out. Hospitalization may be necessary in cases of severe dehydration, fever, neutropenia, or nausea and vomiting that prevents oral hydration (77). Admitted patients should receive intravenous fluid resuscitation, anti-diarrheal agents such as loperamide or octreotide as well as electrolyte supplementation as needed (78). Orally administered, topically active corticosteroid budesonide is active in loperamide resistant chemotherapy induced diarrhea (79,80).
Infusion reactions
The incidence of severe infusion reactions in mCRC patients treated with cetuximab is 3.5–7.5% and with panitumumab <3% (55). The lower incidence with panitumumab as compared with cetuximab is likely due to panitumumab being a fully humanized antibody (81). The mechanisms of infusion and hypersensitivity reactions to the two antibodies may differ, and cases of successful treatment with panitumumab after severe hypersensitivity to cetuximab, as well as vice versa, have been described (82-84).
The administration of corticosteroids (dexamethasone or hydrocortisone) with antihistaminics (diphenhydramine) prior to cetuximab infusion reduces the incidence of infusion reactions, without limiting efficacy (85).
Anti-HER2 agents (trastuzumab)
Based on results from the Trastuzumab for Gastric Cancer (ToGA) trial (86), trastuzumab was approved by the FDA in 2010 in combination with cisplatin and a fluoropyrimidine in patients with Her2/Neu amplified metastatic gastric or gastroesophageal junction adenocarcinoma. Prior to this, trastuzumab had been extensively used in breast cancer, hence its toxicity profile was well characterized much before approval for use in GI malignancies.
In the ToGA trial, there were no significant differences in the incidence of grade 3–4 adverse events upon addition of trastuzumab, except for diarrhea (9% in trastuzumab plus chemotherapy vs. 4% in chemotherapy alone). Additionally, the incidence of grade 3–4 cardiac adverse events was also found to be similar in the two groups (6% in both). Four patients in the trastuzumab/chemotherapy group had cardiac events versus nine patients in the chemotherapy alone group. The incidence of cardiac dysfunction, defined as a ≥10% drop in left ventricular ejection fraction (LVEF) to an absolute value <50%, was 5% in the trastuzumab plus chemotherapy arm versus 1% in the chemotherapy alone arm (86).
The cardiotoxicity of trastuzumab in breast cancer is known to be accentuated when given concurrently with an anthracycline. Concomitant use of trastuzumab and anthracycline (epirubicin) containing regimens in gastric cancer should be avoided. In a large meta-analysis of over 29,000 women, severe cardiotoxicity associated with trastuzumab was seen in 3% of patients (87). Similar to prior studies, the meta-analysis demonstrated an increased rate of severe cardiotoxicity when anthracyclines and trastuzumab were used together versus trastuzumab alone (2.9% versus 0.9%).
The incidence of cardiotoxicity in patients treated with trastuzumab specifically for GI malignancies is not currently characterized, but it is safe to assume that the risk is similar to patients with breast cancer. Trastuzumab associated cardiac toxicity manifests as left ventricular dysfunction, arrhythmias, hypertension, congestive heart failure or cardiomyopathy. Risk factors for cardiotoxicity with trastuzumab therapy are similar to those observed in the general population—pre-existing hypertension, smoking, diabetes, obesity, dyslipidemia, family history of cardiovascular disease, and personal history of coronary artery disease (87,88).
Patients should undergo a thorough cardiac assessment including baseline LVEF prior to initiation of therapy, and every 3 months during and upon completion of trastuzumab therapy. Trastuzumab should be held if there is a ≥16% absolute decrease in LVEF from pre-treatment values or an LVEF value below the institutional limit of normal and ≥10% absolute decrease from pre-treatment value. If trastuzumab has been withheld for significant LV dysfunction, LVEF measurement should be repeated at 4-week intervals.
In the setting of metastatic gastric cancer, the most common (>10%) adverse reactions in the trastuzumab arm compared to control were neutropenia, diarrhea, fatigue, anemia, stomatitis, weight loss, upper respiratory tract infections, fever, thrombocytopenia, mucosal inflammation, nasopharyngitis, and dysgeusia. The most common reactions resulting in discontinuation of treatment were infection, diarrhea and febrile neutropenia.
Immunotherapy agents
Immunotherapy is fast emerging as an effective anti-neoplastic treatment, alternative to chemotherapy for a number of malignancies. Anti-CTLA-4 agents (ipilimumab) and anti-PD1 agents (nivolumab, pembrolizumab) are already approved for use in metastatic melanoma and non-small cell lung cancer (89-91). These results have paved the way for immunotherapy trials in GI malignancies including esophageal, gastric, pancreatic, hepatocellular, colorectal and anal cancer. Though these are not currently approved for use in any GI malignancy, more patients are receiving these agents on clinical trials, and given their unique toxicities, clinicians should be familiar with managing these adverse events.
Immune checkpoint inhibition with anti-CTLA-4 and anti-PD-1/PD-L1 agents triggers a number of autoimmune endocrinopathies affecting the pituitary, thyroid, adrenals, and endocrine pancreas. Autoimmune attacks on non-endocrine sites are also seen resulting in dermatological toxicity, colitis, pneumonitis, hepatitis or myocarditis (92-96). Patients receiving these agents should have regular thyroid function studies, CBC, liver function tests and metabolic panels at each treatment and at 6–12 weeks intervals for 6 months after completion of therapy (97). Table 3 outlines the major immune related adverse events (irAEs) seen with immune checkpoint inhibitors, their incidence and management.
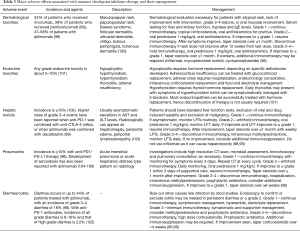
Full table
Other types of immunotherapies under further evaluation are tumor vaccines and adoptive cell transfer therapy (107). Adoptive cell transfer involves the administration of activated, tumor reactive, ex vivo expanded T cells to directly attack cancer cells. This requires a preparative chemotherapy for lymphodepletion which results in transient neutropenia and thrombocytopenia. Administration of active T cells can cause a cytokine release syndrome characterized by fever, tachycardia, oliguria, hypotension and multi-organ failure. Treatment usually involves supportive care with fluids and anti-inflammatory agents while awaiting spontaneous recovery (97). Administration of T-cells can result in autoimmunity as well, with the clinical manifestations depending on the intended target on the cancer cells. For example, when carcinoembryonic antigen was targeted for mCRC, severe life threatening colitis was seen (108).
An oncolytic virus based vaccine approved for metastatic melanoma—talimogene laherparepvec (TVEC)—also has a favourable toxicity profile, with the only ≥ grade 3 toxicity in >2% of patients being cellulitis (109), and is currently undergoing clinical trials in GI malignancies as well.
Conclusions
Biological agents are an indispensable addition to chemotherapy for GI malignancies leading to improved response rates and overall survival. However, the addition of these novel drugs brings forth a number of unique adverse events in addition to those already seen with combination chemotherapeutic regimens. It is important for the care team, including patients and their caregivers, surgeons, nurses, oncologists and primary care physicians to be able to recognize these adverse events to allow for prompt referral and optimal management and lower the risk of permanent sequelae.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. SEER Cancer Statistics Review (CSR) 1975-2013. Available online: http://seer.cancer.gov/csr/1975_2013/
- Rodgers KR, Chou RC. Therapeutic monoclonal antibodies and derivatives: Historical perspectives and future directions. Biotechnol Adv 2016;34:1149-58. [Crossref] [PubMed]
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010;10:317-27. [Crossref] [PubMed]
- Gaya A, Tse V. A preclinical and clinical review of aflibercept for the management of cancer. Cancer Treat Rev 2012;38:484-93. [Crossref] [PubMed]
- Greig SL, Keating GM. Ramucirumab: A Review in Advanced Gastric Cancer. BioDrugs 2015;29:341-51. [Crossref] [PubMed]
- Qi WX, Shen Z, Tang LN, et al. Risk of hypertension in cancer patients treated with aflibercept: a systematic review and meta-analysis. Clin Drug Investig 2014;34:231-40. [Crossref] [PubMed]
- Verdaguer H, Tabernero J, Macarulla T. Ramucirumab in metastatic colorectal cancer: evidence to date and place in therapy. Ther Adv Med Oncol 2016;8:230-42. [Crossref] [PubMed]
- Qu CY, Zheng Y, Zhou M, et al. Value of bevacizumab in treatment of colorectal cancer: A meta-analysis. World J Gastroenterol 2015;21:5072-80. [Crossref] [PubMed]
- Shirakawa T, Nakano M, Nio K, et al. Retrospective analysis of cardiovascular diseases related to chemotherapies for advanced solid tumor patients. Anticancer Drugs 2016;27:891-8. [Crossref] [PubMed]
- Chen ZI, Ai DI. Cardiotoxicity associated with targeted cancer therapies. Mol Clin Oncol 2016;4:675-81. [PubMed]
- Schneider BP, Li L, Shen F, et al. Genetic variant predicts bevacizumab-induced hypertension in ECOG-5103 and ECOG-2100. Br J Cancer 2014;111:1241-8. [Crossref] [PubMed]
- Lambrechts D, Moisse M, Delmar P, et al. Genetic markers of bevacizumab-induced hypertension. Angiogenesis 2014;17:685-94. [PubMed]
- Abbas A, Mirza MM, Ganti AK, et al. Renal Toxicities of Targeted Therapies. Target Oncol 2015;10:487-99. [Crossref] [PubMed]
- Souza VB, Silva EN, Ribeiro ML, et al. Hypertension in patients with cancer. Arq Bras Cardiol 2015;104:246-52. [PubMed]
- Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 2010;102:596-604. [Crossref] [PubMed]
- Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther 2006;28:1779-802. [Crossref] [PubMed]
- Economopoulou P, Kotsakis A, Kapiris I, et al. Cancer therapy and cardiovascular risk: focus on bevacizumab. Cancer Manag Res 2015;7:133-43. [Crossref] [PubMed]
- Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: safety profile and management of adverse events. Semin Oncol 2006;33:S26-34. [Crossref] [PubMed]
- Cosmai L, Gallieni M, Liguigli W, et al. Renal toxicity of anticancer agents targeting vascular endothelial growth factor (VEGF) and its receptors (VEGFRs). J Nephrol 2017;30:171-80. [Crossref] [PubMed]
- Hanna RM, Lopez E, Wilson J, et al. Minimal change disease onset observed after bevacizumab administration. Clin Kidney J 2016;9:239-44. [Crossref] [PubMed]
- Pérez-Valdivia MA, López-Mendoza M, Toro-Prieto FJ, et al. Relapse of minimal change disease nephrotic syndrome after administering intravitreal bevacizumab. Nefrologia 2014;34:421-2. [PubMed]
- Hurwitz HI, Saltz LB, Van Cutsem E, et al. Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol 2011;29:1757-64. [Crossref] [PubMed]
- Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 2007;99:1232-9. [Crossref] [PubMed]
- Okines AF, Langley RE, Thompson LC, et al. Bevacizumab with peri-operative epirubicin, cisplatin and capecitabine (ECX) in localised gastro-oesophageal adenocarcinoma: a safety report. Ann Oncol 2013;24:702-9. [Crossref] [PubMed]
- Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist 2009;14:862-70. [Crossref] [PubMed]
- Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 2007;96:1788-95. [Crossref] [PubMed]
- Brandes AA, Bartolotti M, Tosoni A, et al. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist 2015;20:166-75. [Crossref] [PubMed]
- Cartwright TH. Adverse events associated with antiangiogenic agents in combination with cytotoxic chemotherapy in metastatic colorectal cancer and their management. Clin Colorectal Cancer 2013;12:86-94. [Crossref] [PubMed]
- Qi WX, Shen Z, Tang LN, et al. Bevacizumab increases the risk of gastrointestinal perforation in cancer patients: a meta-analysis with a focus on different subgroups. Eur J Clin Pharmacol 2014;70:893-906. [Crossref] [PubMed]
- Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 2009;10:559-68. [Crossref] [PubMed]
- Qi WX, Shen F, Qing Z, et al. Risk of gastrointestinal perforation in cancer patients treated with aflibercept: a systematic review and meta-analysis. Tumour Biol 2014;35:10715-22. [Crossref] [PubMed]
- Badgwell BD, Camp ER, Feig B, et al. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol 2008;19:577-82. [Crossref] [PubMed]
- Bates DO, Jones RO. The role of vascular endothelial growth factor in wound healing. Int J Low Extrem Wounds 2003;2:107-20. [Crossref] [PubMed]
- Nissen NN, Polverini PJ, Koch AE, et al. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 1998;152:1445-52. [PubMed]
- Bao P, Kodra A, Tomic-Canic M, et al. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153:347-58. [Crossref] [PubMed]
- Gordon CR, Rojavin Y, Patel M, et al. A review on bevacizumab and surgical wound healing: an important warning to all surgeons. Ann Plast Surg 2009;62:707-9. [Crossref] [PubMed]
- Tabernero J, Van Cutsem E, Lakomy R, et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer 2014;50:320-31. [Crossref] [PubMed]
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499-506. [Crossref] [PubMed]
- Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 2015;16:499-508. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Graham J, Muhsin M, Kirkpatrick P. Cetuximab. Nat Rev Drug Discov 2004;3:549-50. [Crossref] [PubMed]
- Lo L, Patel D, Townsend AR, et al. Pharmacokinetic and pharmacodynamic evaluation of panitumumab in the treatment of colorectal cancer. Expert Opin Drug Metab Toxicol 2015;11:1907-24. [Crossref] [PubMed]
- Peeters M, Balfour J, Arnold D. Review article: panitumumab--a fully human anti-EGFR monoclonal antibody for treatment of metastatic colorectal cancer. Aliment Pharmacol Ther 2008;28:269-81. [Crossref] [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [Crossref] [PubMed]
- Saltz LB, Meropol NJ, Loehrer PJ Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 2004;22:1201-8. [Crossref] [PubMed]
- Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040-8. [Crossref] [PubMed]
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658-64. [Crossref] [PubMed]
- Berlin J, Posey J, Tchekmedyian S, et al. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin Colorectal Cancer 2007;6:427-32. [Crossref] [PubMed]
- Hecht JR, Patnaik A, Berlin J, et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer 2007;110:980-8. [Crossref] [PubMed]
- Jaka A, Gutierrez-Rivera A, Lopez-Pestana A, et al. Predictors of Tumor Response to Cetuximab and Panitumumab in 116 Patients and a Review of Approaches to Managing Skin Toxicity. Actas Dermosifiliogr 2015;106:483-92. [Crossref] [PubMed]
- Mitchell EP, Perez-Soler R, Van Cutsem E, et al. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology (Williston Park) 2007;21:4-9. [PubMed]
- Lacouture ME, Mitchell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:1351-7. [Crossref] [PubMed]
- Petrelli F, Borgonovo K, Cabiddu M, et al. Antibiotic prophylaxis for skin toxicity induced by antiepidermal growth factor receptor agents: a systematic review and meta-analysis. Br J Dermatol 2016;175:1166-74. [Crossref] [PubMed]
- Pinto C, Barone CA, Girolomoni G, et al. Management of Skin Reactions During Cetuximab Treatment in Association With Chemotherapy or Radiotherapy: Update of the Italian Expert Recommendations. Am J Clin Oncol 2016;39:407-15. [Crossref] [PubMed]
- Fakih M, Vincent M. Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr Oncol 2010;17 Suppl 1:S18-30. [PubMed]
- Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol 2009;16:16-26. [Crossref] [PubMed]
- Agero AL, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 2006;55:657-70. [Crossref] [PubMed]
- Perez-Soler R. Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer 2006;8 Suppl 1:S7-14. [Crossref] [PubMed]
- Porzio G, Aielli F, Verna L, et al. Efficacy of pregabalin in the management of cetuximab-related itch. J Pain Symptom Manage 2006;32:397-8. [Crossref] [PubMed]
- Peuvrel L, Dreno B. Dermatological toxicity associated with targeted therapies in cancer: optimal management. Am J Clin Dermatol 2014;15:425-44. [Crossref] [PubMed]
- Robert C, Sibaud V, Mateus C, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol 2015;16:e181-9. [Crossref] [PubMed]
- Wnorowski AM, de Souza A, Chachoua A, et al. The management of EGFR inhibitor adverse events: a case series and treatment paradigm. Int J Dermatol 2012;51:223-32. [Crossref] [PubMed]
- Ouwerkerk J, Boers-Doets C. Best practices in the management of toxicities related to anti-EGFR agents for metastatic colorectal cancer. Eur J Oncol Nurs 2010;14:337-49. [Crossref] [PubMed]
- Borkar DS, Lacouture ME, Basti S. Spectrum of ocular toxicities from epidermal growth factor receptor inhibitors and their intermediate-term follow-up: a five-year review. Support Care Cancer 2013;21:1167-74. [Crossref] [PubMed]
- Jiang DM, Dennis K, Steinmetz A, et al. Management of Epidermal Growth Factor Receptor Inhibitor-Induced Hypomagnesemia: A Systematic Review. Clin Colorectal Cancer 2016;15:e117-23. [Crossref] [PubMed]
- Van Cutsem E, Siena S, Humblet Y, et al. An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol 2008;19:92-8. [Crossref] [PubMed]
- Tejpar S, Piessevaux H, Claes K, et al. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol 2007;8:387-94. [Crossref] [PubMed]
- Fakih MG, Wilding G, Lombardo J. Cetuximab-induced hypomagnesemia in patients with colorectal cancer. Clin Colorectal Cancer 2006;6:152-6. [Crossref] [PubMed]
- Schrag D, Chung KY, Flombaum C, et al. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst 2005;97:1221-4. [Crossref] [PubMed]
- Fakih M. Anti-EGFR monoclonal antibody-induced hypomagnesaemia. Lancet Oncol 2007;8:366-7. [Crossref] [PubMed]
- Martin KJ, Gonzalez EA, Slatopolsky E. Clinical consequences and management of hypomagnesemia. J Am Soc Nephrol 2009;20:2291-5. [Crossref] [PubMed]
- Raoul JL, Van Laethem JL, Peeters M, et al. Cetuximab in combination with irinotecan/5-fluorouracil/folinic acid (FOLFIRI) in the initial treatment of metastatic colorectal cancer: a multicentre two-part phase I/II study. BMC Cancer 2009;9:112. [Crossref] [PubMed]
- Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663-71. [Crossref] [PubMed]
- Saltz LB, Lenz HJ, Kindler HL, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol 2007;25:4557-61. [Crossref] [PubMed]
- Tol J, Koopman M, Rodenburg CJ, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol 2008;19:734-8. [Crossref] [PubMed]
- Miroddi M, Sterrantino C, Simonelli I, et al. Risk of grade 3-4 diarrhea and mucositis in colorectal cancer patients receiving anti-EGFR monoclonal antibodies regimens: A meta-analysis of 18 randomized controlled clinical trials. Crit Rev Oncol Hematol 2015;96:355-71. [Crossref] [PubMed]
- Maroun JA, Anthony LB, Blais N, et al. Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. Curr Oncol 2007;14:13-20. [Crossref] [PubMed]
- Andreyev J, Ross P, Donnellan C, et al. Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol 2014;15:e447-60. [Crossref] [PubMed]
- Karthaus M, Ballo H, Abenhardt W, et al. Prospective, double-blind, placebo-controlled, multicenter, randomized phase III study with orally administered budesonide for prevention of irinotecan (CPT-11)-induced diarrhea in patients with advanced colorectal cancer. Oncology 2005;68:326-32. [Crossref] [PubMed]
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009;15:5591-8. [Crossref] [PubMed]
- Del Prete M, Giampieri R, Faloppi L, et al. Panitumumab for the treatment of metastatic colorectal cancer: a review. Immunotherapy 2015;7:721-38. [Crossref] [PubMed]
- Saif MW, Peccerillo J, Potter V. Successful re-challenge with panitumumab in patients who developed hypersensitivity reactions to cetuximab: report of three cases and review of literature. Cancer Chemother Pharmacol 2009;63:1017-22. [Crossref] [PubMed]
- Saif MW, Syrigos KI, Hotchkiss S, et al. Successful desensitization with cetuximab after an infusion reaction to panitumumab in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol 2009;65:107-12. [Crossref] [PubMed]
- Heun J, Holen K. Treatment with panitumumab after a severe infusion reaction to cetuximab in a patient with metastatic colorectal cancer: a case report. Clin Colorectal Cancer 2007;6:529-31. [Crossref] [PubMed]
- Siena S, Glynne-Jones R, Adenis A, et al. Reduced incidence of infusion-related reactions in metastatic colorectal cancer during treatment with cetuximab plus irinotecan with combined corticosteroid and antihistamine premedication. Cancer 2010;116:1827-37. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Mantarro S, Rossi M, Bonifazi M, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg Med 2016;11:123-40. [Crossref] [PubMed]
- Sandoo A, Kitas GD, Carmichael AR. Breast cancer therapy and cardiovascular risk: focus on trastuzumab. Vasc Health Risk Manag 2015;11:223-8. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Specenier P. Ipilimumab in melanoma. Expert Rev Anticancer Ther 2016;16:811-26. [Crossref] [PubMed]
- Giri A, Walia SS, Gajra A. Clinical Trials Investigating Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer. Rev Recent Clin Trials 2016;11:297-305. [Crossref] [PubMed]
- Mehta A, Gupta A, Hannallah F, et al. Myocarditis as an immune-related adverse event with ipilimumab/nivolumab combination therapy for metastatic melanoma. Melanoma Res 2016;26:319-20. [Crossref] [PubMed]
- Gupta A, De Felice KM, Loftus EV Jr, et al. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther 2015;42:406-17. [Crossref] [PubMed]
- De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res 2015;25:321-7. [Crossref] [PubMed]
- Chow LQ. Exploring novel immune-related toxicities and endpoints with immune-checkpoint inhibitors in non-small cell lung cancer. Am Soc Clin Oncol Educ Book 2013. Available online: http://dx.doi.org/ [Crossref]
- Kähler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges 2016;14:662-81. [PubMed]
- Weber JS, Yang JC, Atkins MB, et al. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol 2015;33:2092-9. [Crossref] [PubMed]
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2016;27:1362. [Crossref] [PubMed]
- Weber JS. Practical management of immune-related adverse events from immune checkpoint protein antibodies for the oncologist. Am Soc Clin Oncol Educ Book 2012.174-7. [PubMed]
- González N, Ratner D. Novel melanoma therapies and their side effects. Cutis 2016;97:426-8. [PubMed]
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. [Crossref] [PubMed]
- Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190-209. [Crossref] [PubMed]
- Kim KW, Ramaiya NH, Krajewski KM, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs 2013;31:1071-7. [Crossref] [PubMed]
- Reule RB, North JP. Cutaneous and pulmonary sarcoidosis-like reaction associated with ipilimumab. J Am Acad Dermatol 2013;69:e272-3. [Crossref] [PubMed]
- Berthod G, Lazor R, Letovanec I, et al. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol 2012;30:e156-9. [Crossref] [PubMed]
- Vogel WV, Guislain A, Kvistborg P, et al. Ipilimumab-induced sarcoidosis in a patient with metastatic melanoma undergoing complete remission. J Clin Oncol 2012;30:e7-e10. [Crossref] [PubMed]
- Singh PP, Sharma PK, Krishnan G, et al. Immune checkpoints and immunotherapy for colorectal cancer. Gastroenterol Rep (Oxf) 2015;3:289-97. [PubMed]
- Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011;19:620-6. [Crossref]
- Ott PA, Hodi FS. Talimogene Laherparepvec for the Treatment of Advanced Melanoma. Clin Cancer Res 2016;22:3127-31. [Crossref] [PubMed]


