Adjuvant therapy in biliary tract and gall bladder carcinomas: a review
Introduction
Cancers of the biliary tract are a relatively rare and aggressive disease that generally present at a locally advanced stage. However, certain geographic areas have a higher incidence of these diseases, and they may be increasing in incidence. The cancer is most commonly adenocarcinoma, arising from the epithelium of the gallbladder, intrahepatic, or extrahepatic biliary ducts. Extrahepatic cholangiocarcinoma (EHCC) is sub-classified anatomically as hilar or distal common bile duct (CBD), usually depending on the relation to the cystic duct insertion. The majority of cholangiocarcinoma is hilar (60–70%), followed by distal CBD (20–30%), and intrahepatic (5–15%) (1).
Risk factors for cholangiocarcinoma are generally related to inflammation and include primary sclerosing cholangitis, chronic choledocolithiasis, and liver fluke infections. Risk factors for gallbladder cancer (GBC) are also generally inflammation mediated and include chronic cholelithiasis, gallbladder calcification (porcelain gallbladder), gallbladder polyps, and inflammatory bowel disease (2,3).
Upfront surgical resection is the mainstay of therapy in patients who are anatomically and medically fit for surgery (4). Approximately 65% of patients who undergo surgical exploration with hilar cholangiocarcinoma will have resectable disease. However this rate is less than 50% if resectable is defined as curative surgery with negative margins (R0) (5). Resectability and overall survival (OS) are well stratified by TNM stage at presentation with 5-year OS for AJCC 7th edition stage I EHCC of 30%, 24% for stage II–III, and 2% for stage IV (6). Stages for stage outcomes are worse for intrahepatic cholangiocarcinoma (ICC).
A predominant pattern of recurrence in patients with biliary cancers is through hematogenous spread of metastatic disease to liver and lung, and to locoregional and distant lymph nodes (LN). Among the difficulties of determining the optimal therapy for biliary cancers is the relative infrequency and the heterogeneity of these diseases. NCCN guidelines offer several options for GBC or cholangiocarcinoma status post resection. These options include observation, adjuvant chemotherapy, or adjuvant chemoradiation (CRT) (4). Due to the relatively rare nature of these cancers, level I evidence based on randomized controlled trials is generally not available to guide therapy decisions in the adjuvant setting. Treatment recommendations must be based on lower levels of evidence, most commonly single institution retrospective studies.
The purpose of this review is to summarize the existing published literature for the adjuvant management of GBC and cholangiocarcinoma and examine the relative merits of these data in shaping the current standards of care.
GBC
GBC is the most common type of biliary tract cancer. Outcomes depend highly on stage with 5-year OS of 60%, 39%, and 15%, 5%, and 1% for stages 0, I, II, III, and IV, respectively (7). The standard of care is initial resection with cholecystectomy, en bloc hepatic resection, and lymphadenectomy with the goal of R0 resection. Patients with pT1N0 tumors are recommended to be observed. Options for adjuvant therapy post resection of pT2 or above or LN positive disease include fluoropyrimidine based CRT, fluoropyrimidine or gemcitabine based chemotherapy, or observation (4) (Table 1).
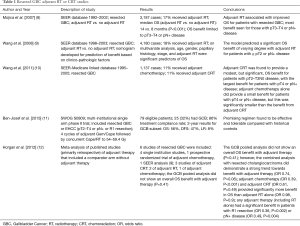
Full table
There is evidence that the patterns of failure for resected GBC may be distinct from that of EHCC. A single institution retrospective study of 177 patients, of whom 97 (55%) had GBC and 80 (45%) had hilar cholangiocarcinoma, reported patterns of first recurrence (13). The minority of patients (11%) received any adjuvant therapy. Overall recurrence was higher and time to first recurrence was shorter for GBC compared with hilar cholangiocarcinoma. Of patients with recurrence, isolated locoregional recurrence (LRR) as 1st recurrence occurred in 15% of patients with GBC compared with 59% of those with hilar cholangiocarcinoma (P<0.001). Distant recurrence (with or without LRR) occurred in 85% of patients with GBC compared with 41% of those with hilar cholangiocarcinoma (P<0.001). Primary site was an independent predictor of site of initial recurrence controlling for other clinico-pathologic factors.
Adjuvant chemotherapy
The strongest data in support of adjuvant systemic therapy for any of the biliary tract carcinomas is derived from a Japanese study reported by Takada et al., and more specifically applies to gall bladder adenocarcinomas (14). In this study, a heterogenous group of 508 patients with pancreatic, ampullary, biliary, and gall bladder cancers, underwent resection between 1986 and 1992. One hundred and forty patients with gall bladder cancers were enrolled. Patients were randomly assigned to undergo observation, or to receive adjuvant therapy with Mitomycin C 6 mg/m2 on the day of surgery, then 5-fluorouracil (5-FU) 310 mg/m2 IV daily for 5 days on week 1 and 3 postoperatively, then oral 5-FU 100 mg/m2 daily starting in week 5. Overall, the study failed to demonstrate a survival benefit at 5 years, the primary endpoint, and the only patients that did demonstrate a significant survival benefit were those with gall bladder cancer. The 5-year OS of patients with gall bladder cancer receiving adjuvant therapy was 26.0%, compared to 14.4% in those who were observed.
In addition, a meta-analysis that focused specifically on gall bladder cancers demonstrated that adjuvant chemotherapy did improve survival compared to surgery alone (15). However, an evaluation of the National Cancer Database (NCDB) did not demonstrate a survival benefit with adjuvant chemotherapy (16).
Adjuvant RT/CRT
Due to the relatively small number of GBC cases, the current standards of care are based primarily on population based analyses utilizing Surveillance, Epidemiology, and End Results (SEER) data.
An initial study using the SEER database for patients with GBC diagnosed between 1992 and 2002 was reported in 2007 (8). SEER does not contain chemotherapy information; hence this was a study of adjuvant RT (concurrent chemotherapy use unknown) versus no adjuvant RT. A total of 3,187 cases were identified, of which 17% received adjuvant RT. Median OS was 14 months compared with 8 months (P<0.001) for those who did and did not receive RT, respectively. Subset analysis according to disease extent found the OS benefit with RT was limited to those with regional spread (LN positive disease) or liver involvement (pT3 according to AJCC 7th editions staging).
A SEER study with a largely overlapping patient population (incident cases from 1988–2003) was published in 2008 (9). This study generated nomograms for prediction of short term OS with and without adjuvant RT. On multivariate analysis, age, gender, papillary histology, stage, and adjuvant RT were significant predictors of OS. The model predicted a significant OS benefit with adjuvant RT for patients with ≥ pT2N+ (LN positive) disease. Patients with pT1 disease did not benefit regardless of LN status and those with ≥ pT2N0 disease had variable benefit that was dependent on other model variables.
A follow-up study published in 2011 by the same group used SEER-Medicare linked data, which contained information on both RT and chemotherapy use (10). A total of 1,137 patients with GBC resected between 1995 and 2005 were included. A minority of patients received either adjuvant chemotherapy (11%) or CRT (11%). The primary end point was OS with or without adjuvant chemotherapy or CRT. Adjuvant CRT was found to provide a modest, but significant, OS benefit for patients with pT2–T3N0 disease, with the largest benefit for patients with pT4 or pN+ disease. Adjuvant chemotherapy alone did provide a small benefit for patients with pT4 or pN+ disease, but this was significantly smaller than the benefit from adjuvant CRT. These results were partially mirrored in a retrospective Korean study of 100 patients with resected GCB (17). They found a significant benefit in disease free survival (DFS) and disease specific survival (DSS) for adjuvant CRT for patients with pT2−T3N+, but no benefit for pT2-T3N0.
A recently published prospective multi-institutional phase II trial (SWOG S0809) included patients with resected GBC or EHCC, pT2-T4 or pN+ or R1 resection status (11). Treatment consisted of 4 cycles of gemcitabine and capecitabine followed by concurrent capecitabine with RT to a total dose of 54–59.4 Gy. There were 79 eligible patients, of which 25 (32%) had GCB. Treatment compliance was high with 86% of patients completing therapy. The 2-year OS, DFS, and local recurrence (LR) rates were 56%, 47%, and 8% for GBC subset, respectively. These outcomes did not differ significantly from the EHCC subset. This regimen was found to be effective, tolerable, and a promising adjuvant regimen compared with historical controls.
A meta-analysis of primarily retrospective studies of adjuvant therapy that included a comparator arm without adjuvant therapy was published in 2012 (12). Six studies of patients with resected GBC were included, of which four were single institution retrospective studies and 1 was a randomized trial of adjuvant chemotherapy alone, all of which consisted of a total of 270 patients. The 6th study was the aforementioned SEER study by Wang et al. of 4,180 patients (9). Three studies were of adjuvant CRT, 2 reported on adjuvant RT alone, and 1 was of adjuvant chemotherapy. The GCB pooled analysis did not show an overall OS benefit with adjuvant therapy (P=0.41). However, the combined analysis with resected cholangiocarcinoma did demonstrate a strong trend towards benefit with adjuvant therapy [odds ratio (OR) 0.74, P=0.06]. When separated by treatment modality, adjuvant chemotherapy (OR 0.39, P<0.001) and adjuvant CRT (OR 0.61, P=0.49) provided significantly more benefit in OS than adjuvant RT alone (OR 0.98, P=0.9, significant treatment interaction by modality, P=0.02). Any adjuvant therapy (including RT alone) had a significant benefit in patients with R1 resection (OR 0.36, P=0.002) or pN+ disease (OR 0.49, P=0.004).
In the context of the other aforementioned studies, these data support the use of adjuvant therapy (chemotherapy or CRT) for patients with ≥ pT2, pN+, or R1 resected GBC, with the most benefit in those with R1 resection or pN+ disease.
Biliary tract carcinoma
Adjuvant chemotherapy
Most studies evaluating the potential benefits of adjuvant chemotherapy in biliary tract carcinomas included all anatomic sites, limiting the power of the conclusions that can be drawn. For example, the trial reported by Takada et al. included pancreatic, ampullary and biliary carcinomas as well as gall bladder carcinomas (14). While there was a suggestion of benefit in the group of patients with gall bladder carcinomas, the study did not demonstrate a survival benefit overall. Since ESPAC-1 and CONKO-001 both demonstrated a survival benefit for adjuvant chemotherapy with 5-FU/Leucovorin and Gemcitabine, respectively, in pancreatic adenocarcinomas, these results suggest that this type of regimen may be ineffective in biliary and ampullary carcinomas (18,19).
A lack of benefit of adjuvant chemotherapy is reinforced by a subgroup of 428 patients from the ESPAC-3 study with carcinoma of the bile duct, ampulla and periampullary duodenum (20). Most had ampullary carcinomas, and only 96 had biliary carcinomas. Patients were randomly assigned to observation, 5-FU/Leucovorin on a Mayo Clinic schedule (bolus 5-FU and leucovorin for five consecutive days, with cycles repeated every four weeks), or gemcitabine. Since the ESPAC-3 overall analysis did not demonstrate a difference in survival outcomes between the two chemotherapy regimens, the chemotherapy groups were assessed together. While the survival was longer in patients who were treated with chemotherapy (median 43.1 months, compared to 35.2 months with observation), the difference was not statistically significant [hazard ratio (HR) 0.86, P=0.25].
Because of the limited category A level evidence to help guide management recommendations and decisions in biliary tract cancer, Horgan et al. performed a meta-analysis on studies that were published through 2010 (12). The published reports including retrospective studies on patients, and included a total of 6,712 patients, that comprised 4,915 patients who underwent surgery alone, and 1,297 who had received chemotherapy, and/or radiation. The investigators reported that overall, there was no survival benefit with adjuvant therapy. This was especially true for patients who were treated with radiation alone (OR 0.98), but patients who received chemotherapy with (OR 0.61, P=0.049) and without radiation (OR 0.39, P<0.0001) did have improved survival compared to surgery alone.
EHCC
EHCC are adenocarcinomas that arise from the biliary tree, outside of the liver parenchyma. They are further subdivided into hilar and distal CBD cholangiocarcinomas based on their location relative to the cystic duct insertion. EHCC are significantly more common than intrahepatic, though both are relatively rare compared with other cancers of the GI tract.
Expected outcomes for EHCC also depend highly on stage and resectability, with 5-year OS of 30%, 24%, and 2% for local disease (stage I), regional spread (stages II–III), and distant spread (stage IV), respectively (http://www.cancer.org/cancer/bileductcancer/detailedguide/bile-duct-cancer-survival-by-stage). Hilar cholangiocarcinoma can be staged according to AJCC 7th edition (6) or more specialized local staging systems, including modified Bismuth et al. (21) and Blumgart staging systems (22).
Margin negative surgical resection is the mainstay of initial therapy for resectable patients. The typical surgery for hilar cholangiocarcinoma is extended hepatectomy while pancreaticoduodenectomy (PD) is utilized for distal EHCC (23). Negative margins are a major prognostic factor, with some evidence that widely negative margins (≥1 cm) confer even more benefit in terms of long term survival (24). As opposed to GCB, the primary pattern of failure for resected EHCC is loco-regional (13).
In the setting of R0 resection and negative LN, NCCN guidelines recommend observation, fluoropyrimidine based CRT, or fluoropyrimidine or gemcitabine based chemotherapy at the same level 2A recommendation (4). In the setting of incomplete resection (R1 or R2) or positive LN, the recommendations are fluoropyrimidine based CRT followed by fluoropyrimidine or gemcitabine based chemotherapy or fluoropyrimidine or gemcitabine based chemotherapy alone if R0 and pN+.
Adjuvant RT/CRT
No randomized trials have been performed investigating the role or optimal regimen for adjuvant therapy for resected EHCC. There have been many small single institutional retrospective studies over several decades that shape our current understanding. We will focus on the selected studies that form the basis of the current standards of care for adjuvant therapy in resected EHCC (Table 2).
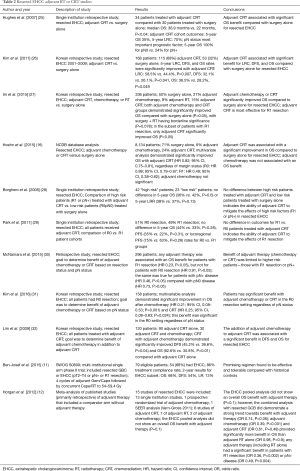
Full table
Several retrospective studies have investigated the use of surgery with adjuvant CRT compared with a historical cohort of surgery alone. A study from Johns Hopkins University included 34 cases of adenocarcinoma of the distal CBD treated with PD with adjuvant CRT (25). The 5-year OS was 35%, with LN status being the most important prognostic factor (5-year OS 100% for pN0 vs. 24% for pN+). Five-year local control was 70%, with all patient deaths due to progressive metastatic disease. Median OS was significantly longer in this cohort treated with PD and adjuvant CRT compared with the historical control of 30 patients treated with PD alone (36.9 vs. 22 months, P<0.04). A retrospective study from Korea included patients with resected EHCC treated with or without adjuvant CRT in the same 2001–2009 time period (26). Of the 168 patients, 115 received adjuvant CRT (68%) and 53 (32%) did not. Five-year LRC, DFS, and OS rates were significantly improved with adjuvant CRT (58.5% vs. 44.4%, P=0.007; 32.1% vs. 26.1%, P=0.041; 36.5% vs. 28.2%, P=0.049, respectively). Adjuvant CRT remained a significant independent prognostic factor for LRC, DFS, and OS on multivariable analysis (P<0.05). A similar, but larger analysis of 336 Korean patients was published in 2015 (27). Patients with resected EHCC were treated with surgery alone (50%), adjuvant chemotherapy (27%), adjuvant RT (9%), or adjuvant CRT (15%). Both surgery with adjuvant chemotherapy or CRT groups demonstrated significantly improved OS compared with surgery alone (P<0.05), with surgery + RT having borderline significance (P=0.078). In the subset of patients with R1 resection, surgery followed by CRT significantly improved OS (P<0.05).
Several population based studies have been published to help inform this clinical question. Two studies published outcomes using the same SEER dataset. The first was published in 2009 and included 1,569 cases diagnosed between 1973 and 2005 (33). Patients treated with surgery and adjuvant RT had improved median OS compared with those receiving either surgery or RT alone (median OS 26, 25, and 12 months, respectively, P<0.001), and all treatment groups had superior OS compared to patients who received no therapy (median OS 9 months). A second study utilizing the same SEER dataset was published in 2011 (34). A total of 1,491 patients diagnosed between 1973 and 2003 were included. Patients with localized disease had significantly improved OS compared to those with regional disease, with median OS of 33 and 18 months, respectively (P<0.001). However, in contrast to the prior SEER study, the addition of adjuvant RT was not associated with benefit in OS or CSS. Importantly, information on margin status and chemotherapy use is not available in the SEER database. A recent study using the NCDB of 8,134 patients treated between 1998 and 2006 was published in 2015 (16). This study compared surgery alone (71%), adjuvant chemotherapy (6%), and adjuvant CRT groups (24%). Information on margin status, demographic data, chemotherapy use, and treatment center type was available. Multivariate analysis demonstrated significantly improved OS with adjuvant CRT (HR 0.82; 95% confidence interval (CI), 0.75–0.91], regardless of margin status (R0: HR 0.88; 95% CI, 0.79–0.97; R1: HR 0.49; 95% CI, 0.38–0.62).
Several retrospective studies have investigated the ability of adjuvant CRT to mitigate the effects of negative prognostic factors in high risk patients with resected EHCC. A single institutional retrospective study from MD Anderson compared patients with R1 or pN+ disease treated with adjuvant CRT (n=42) to those with R0pN0 disease treated with surgery alone (n=23) (28). Patients in the CRT and surgery alone groups had similar 5-year OS (36% vs. 42%, P=0.6) and LRR (38% vs. 37%, P=0.13), indicating the potential ability of adjuvant CRT to mitigate the negative effects of R1 and/or pN+ disease. A study from Korea included 101 patients with resected EHCC who all received adjuvant CRT, with 51% having an R0 resection and 49% having an R1 resection (29). There was no difference in 5-year OS (44% vs. 33%, P=0.28), PFS (35% vs. 22%, P=0.31), or locoregional PFS (75% vs. 63%, P=0.28) rates for R0 vs. R1 groups.
There is conflicting evidence as to if there are patient subgroups who specifically do or do not benefit from adjuvant therapy based on certain risk factors. A retrospective study of 296 patients compared the benefit of adjuvant chemotherapy or CRT for patients based on margin status and LN status (30). Any adjuvant therapy was associated with an OS benefit for patients with R1 resection (HR 0.23, P<0.05), but not for patients with R0 resection (HR 0.91, P>0.05). The same was true for patients with pN+ disease (HR 0.46, P<0.05) compared with pN0 disease (HR 0.73, P>0.05). A Korean retrospective study of 158 patients’ status post R0 resection of EHCC also examined the role of adjuvant therapy (31). Converse to the previous study, multivariable analysis demonstrated significant improvement in OS after chemotherapy (HR 0.21; 95% CI, 0.08–0.53; P=0.001) and CRT (HR 0.25; 95% CI, 0.08–0.83; P=0.024). This benefit was significant in the R0 setting regardless of pN status.
Several studies have investigated the use of both adjuvant CRT and chemotherapy. A retrospective study from Korea included 120 patients with resected EHCC who received adjuvant CRT with (n=90) or without (n=30) additional adjuvant chemotherapy (32). CRT with adjuvant chemotherapy demonstrated significantly improved DFS (45.2% vs. 26.6%, P=0.04) and OS (62.6% vs. 30.8%, P<0.01) compared with adjuvant CRT alone. A recently published prospective multi-institutional phase II trial (SWOG S0809) included patients with resected GBC or EHCC, pT2-T4 or pN+ or R1 resection status (11). Treatment consisted of four cycles of gemcitabine and capecitabine followed by concurrent capecitabine with RT to a total dose of 54–59.4 Gy. There were 79 eligible patients, of which 54 (68%) had EHCC. Treatment compliance was high with 86% of patients completing therapy. The 2-year OS, DFS, and LR rates were 68%, 54%, and 13% for the EHCC subset, respectively. These outcomes did not differ significantly from the GBC subset. This regimen was found to be effective, tolerable, and a promising adjuvant regimen compared with historical controls.
A meta-analysis of primarily retrospective studies of adjuvant therapy that included a comparator arm without adjuvant therapy was published in 2012 (12). Fifteen studies of patients with resected bile duct cancer were included, of which 13 were single institution retrospective studies and 1 was a randomized trial of adjuvant chemotherapy alone, all of which consisted of a total of 771 patients. The 15th study was the aforementioned SEER study by Vern-Gross et al. of 1,491 patients (34). Six studies were of adjuvant CRT, seven reported on RT alone, and two were of adjuvant chemotherapy. The biliary duct cancer pooled analysis did not show an overall OS benefit with adjuvant therapy (P=0.1). However, the combined analysis with resected GCB did demonstrate a strong trend towards benefit with adjuvant therapy (OR 0.74, P=0.06). When separated by treatment modality, adjuvant chemotherapy (OR 0.39, P<0.001) and adjuvant CRT (OR 0.61, P=0.49) provided significantly more benefit in OS than adjuvant RT alone (OR 0.98, P=0.9, significant treatment interaction by modality, P=0.02). Any adjuvant therapy (including RT alone) had a significant benefit in patients with R1 resection (OR 0.36, P=0.002) or pN+ disease (OR 0.49, P=0.004).
In the context of the other aforementioned studies, these data support the use of adjuvant therapy (chemotherapy or CRT) for patients with resected EHCC, with the combination of CRT and chemotherapy indicated for high risk patients, such as those with R1 resection and/or pN+ status.
ICC
ICC represents the minority of cholangiocarcinoma, making up only 5–15% of cases (1). Survival outcomes are worse for ICC stage for stage compared with EHCC, with median OS not reached for stage I, 53 months for stage II, and 16 months for stage III (6).
Surgery is the mainstay of therapy and generally consists of hepatic resection with portal lymphadenectomy, but only approximately 30% of patients present with operable disease (35). NCCN guidelines recommend observation or fluoropyrimide or gemcitabine based chemotherapy after R0 resection (4). Options after R1 resection are fluoropyrimidine CRT or fluoropyrimide or gemcitabine based chemotherapy. R2 resection portends a poor prognosis and gemcitabine/cisplatin combination chemotherapy is a category 1 recommendation, with locoregional therapy listed as a category 2B recommendation.
Adjuvant chemotherapy
While there have not been any prospective or randomized studies evaluating adjuvant chemotherapy in ICC, a number of retrospective single institution studies have suggested that adjuvant chemotherapy is beneficial in this disease (30,36-38). An analysis of the NCDB also supported the potential benefit of adjuvant chemotherapy (39). Several studies have suggested that patients who have a lower likelihood excreting or clearing gemcitabine, as manifested by RRM1 or hENT1 expression are particularly likely to benefit from adjuvant therapy, possibly representing a useful biomarker (40-42). Nonetheless, taken together, available data provides only modest support for adjuvant chemotherapy for IHCC.
Adjuvant RT/CRT
Even more so than the other biliary tract cancer sites, there is a lack of prospective or randomized evidence to guide recommendations for IHCC. The largest study is a population based investigation using the SEER database (43). A total of 3839 patients with IHCC were included, of which 25% received surgery alone, 10% received RT alone, 7% surgery and adjuvant RT, and 58% no surgery or RT. Surgery and adjuvant RT provided the most benefit for OS (HR 0.40; 95% CI, 0.34–0.47), followed by surgery alone (HR 0.49; 95% CI, 0.44–0.54) and RT alone (HR 0.68; 95% CI, 0.59–0.77), compared with no surgery or RT.
Only 1 of 20 studies included the meta-analysis of adjuvant therapy in resected biliary tract cancer included patients with ICC (12). Of the 92 patients in this study, only 11 had ICC.
Adjuvant chemotherapy and/or RT demonstrated a trend towards improved OS compared with surgery alone in the entire patient population (Median OS 42 vs. 29 months, P=0.07). However, patients with distal EHCC were the subgroup that received the most benefit with adjuvant therapy.
Conclusions
While the past several decades have seen advances in the treatment of many cancers, progress in biliary carcinomas has been slow. This is in part because of the heterogeneity of these diseases, making the development and interpretation of clinical trials difficult. This is especially true in the perioperative therapy of biliary cancers, where for the most part, treatment recommendations are based on retrospective series and expert opinion. Nonetheless, there appears to be some consensus that adjuvant therapy may be warranted in patients with incompletely resected disease, either R1 or R2, and potentially in patients with more advanced disease, particularly nodal involvement. Gemcitabine and fluoropyrimidine-based chemotherapy with or without platinum, and with or without radiation are supported, though none is clearly favored as a therapeutic approach. Certainly more studies with future attention to molecular or biomarker approaches in these diseases are necessary to further advance management recommendations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Anderson CD, Pinson CW, Berlin J, et al. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9:43-57. [Crossref] [PubMed]
- Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg 2007;11:671-81. [Crossref] [PubMed]
- Sheth S, Bedford A, Chopra S. Primary gallbladder cancer: recognition of risk factors and the role of prophylactic cholecystectomy. Am J Gastroenterol 2000;95:1402-10. [Crossref] [PubMed]
- Network NCC. Hepatobiliary Cancers Version 1.2016. Vol 2016: National Comprehensive Cancer Network.
- Nagorney DM, Kendrick ML. Hepatic resection in the treatment of hilar cholangiocarcinoma. Adv Surg 2006;40:159-71. [Crossref] [PubMed]
- Edge SB, American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer, 2010.
- Donohue JH, Stewart AK, Menck HR. The National Cancer Data Base report on carcinoma of the gallbladder, 1989-1995. Cancer 1998;83:2618-28. [Crossref] [PubMed]
- Mojica P, Smith D, Ellenhorn J. Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. J Surg Oncol 2007;96:8-13. [Crossref] [PubMed]
- Wang SJ, Fuller CD, Kim JS, et al. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol 2008;26:2112-7. [Crossref] [PubMed]
- Wang SJ, Lemieux A, Kalpathy-Cramer J, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 2011;29:4627-32. [Crossref] [PubMed]
- Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol 2015;33:2617-22. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689-700. [Crossref] [PubMed]
- Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 2002;95:1685-95. [Crossref] [PubMed]
- Ma N, Cheng H, Qin B, et al. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer 2015;15:615. [Crossref] [PubMed]
- Hoehn RS, Wima K, Ertel AE, et al. Adjuvant Chemotherapy and Radiation Therapy is Associated with Improved Survival for Patients with Extrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22 Suppl 3:S1133-9. [Crossref] [PubMed]
- Cho SY, Kim SH, Park SJ, et al. Adjuvant chemoradiation therapy in gallbladder cancer. J Surg Oncol 2010;102:87-93. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 2012;308:147-56. [Crossref] [PubMed]
- Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992;215:31-8. [Crossref] [PubMed]
- Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-17; discussion 517-9. [Crossref] [PubMed]
- Akamatsu N, Sugawara Y, Hashimoto D. Surgical strategy for bile duct cancer: Advances and current limitations. World J Clin Oncol 2011;2:94-107. [Crossref] [PubMed]
- Glazer ES, Liu P, Abdalla EK, et al. Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J Gastrointest Surg 2012;16:1666-71. [Crossref] [PubMed]
- Hughes MA, Frassica DA, Yeo CJ, et al. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys 2007;68:178-82. [Crossref] [PubMed]
- Kim TH, Han SS, Park SJ, et al. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys 2011;81:e853-9. [Crossref] [PubMed]
- Im JH, Seong J, Lee IJ, et al. Surgery Alone Versus Surgery Followed by Chemotherapy and Radiotherapy in Resected Extrahepatic Bile Duct Cancer: Treatment Outcome Analysis of 336 Patients. Cancer Res Treat 2016;48:583-95. [Crossref] [PubMed]
- Borghero Y, Crane CH, Szklaruk J, et al. Extrahepatic bile duct adenocarcinoma: patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone. Ann Surg Oncol 2008;15:3147-3156. [Crossref] [PubMed]
- Park JH, Choi EK, Ahn SD, et al. Postoperative chemoradiotherapy for extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys 2011;79:696-704. [Crossref] [PubMed]
- McNamara MG, Walter T, Horgan AM, et al. Outcome of adjuvant therapy in biliary tract cancers. Am J Clin Oncol 2015;38:382-7. [Crossref] [PubMed]
- Kim YS, Hwang IG, Park SE, et al. Role of adjuvant therapy after R0 resection for patients with distal cholangiocarcinoma. Cancer Chemother Pharmacol 2016;77:979-85. [Crossref] [PubMed]
- Lim KH, Oh DY, Chie EK, et al. Adjuvant concurrent chemoradiation therapy (CCRT) alone versus CCRT followed by adjuvant chemotherapy: which is better in patients with radically resected extrahepatic biliary tract cancer?: a non-randomized, single center study. BMC Cancer 2009;9:345. [Crossref] [PubMed]
- Fuller CD, Wang SJ, Choi M, et al. Multimodality therapy for locoregional extrahepatic cholangiocarcinoma: a population-based analysis. Cancer 2009;115:5175-83. [Crossref] [PubMed]
- Vern-Gross TZ, Shivnani AT, Chen K, et al. Survival outcomes in resected extrahepatic cholangiocarcinoma: effect of adjuvant radiotherapy in a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys 2011;81:189-98. [Crossref] [PubMed]
- Chou FF, Sheen-Chen SM, Chen YS, et al. Surgical treatment of cholangiocarcinoma. Hepatogastroenterology 1997;44:760-5. [PubMed]
- Sur MD, In H, Sharpe SM, et al. Defining the Benefit of Adjuvant Therapy Following Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:2209-17. [Crossref] [PubMed]
- Wirasorn K, Ngamprasertchai T, Khuntikeo N, et al. Adjuvant chemotherapy in resectable cholangiocarcinoma patients. J Gastroenterol Hepatol 2013;28:1885-91. [Crossref] [PubMed]
- Yamanaka K, Hatano E, Kanai M, et al. A single-center analysis of the survival benefits of adjuvant gemcitabine chemotherapy for biliary tract cancer. Int J Clin Oncol 2014;19:485-9. [Crossref] [PubMed]
- Miura JT, Johnston FM, Tsai S, et al. Chemotherapy for Surgically Resected Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:3716-23. [Crossref] [PubMed]
- Sasaki H, Murakami Y, Uemura K, et al. Concurrent analysis of human equilibrative nucleoside transporter 1 and ribonucleotide reductase subunit 1 expression increases predictive value for prognosis in cholangiocarcinoma patients treated with adjuvant gemcitabine-based chemotherapy. Br J Cancer 2014;111:1275-84. [Crossref] [PubMed]
- Kobayashi H, Murakami Y, Uemura K, et al. Human equilibrative nucleoside transporter 1 expression predicts survival of advanced cholangiocarcinoma patients treated with gemcitabine-based adjuvant chemotherapy after surgical resection. Ann Surg 2012;256:288-96. [Crossref] [PubMed]
- Suzuki S, Kaji S, Koike N, et al. Adjuvant chemotherapy using gemcitabine for resected distal bile duct and ampullary cancers. Hepatogastroenterology 2014;61:314-8. [PubMed]
- Shinohara ET, Mitra N, Guo M, et al. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2008;72:1495-501. [Crossref] [PubMed]


