EBV-associated hepatic smooth muscle tumor of uncertain biologic behavior after heart transplantation in a pediatric patient: case report
Introduction
Epstein-Barr virus (EBV) has been involved in the pathogenesis of different types of malignancies. The most frequently reported EBV-associated malignancies are Burkitt lymphoma, Hodgkin lymphoma, post-transplant lymphoproliferative disorder (PTLD), and nasopharyngeal carcinoma (1-4). Recently, the strong relation of EBV to the development of smooth muscle tumors (SMTs) in immunocompromised patients has been increasingly recognized, mainly in post-transplantation and AIDS patients (5,6). EBV-SMTs are rare neoplasms, with less than 30 cases reported in post solid organ transplantation pediatric patients. They are usually multicentric and exhibit a predilection to occur in sites that are unusual for conventional SMTs, most commonly the liver and lung (5,6).
Herein we present a pediatric case of multiple EBV-SMTs identified first in the liver, and subsequently in the abdominal wall and mesentery, 8 years after heart transplantation.
Case presentation
The patient is an 8-year-old female who received a heart transplant at 6 weeks of age for truncus arteriosus. Initial immunosuppression was achieved with mycophenolate mofetil and cyclosporine, and later changed to tacrolimus. EBV serology was not determined at the time of transplantation. Serial testing for EBV DNA by quantitative polymerase chain reaction (PCR) was performed on peripheral blood starting 3 years after the heart transplant (she presented with cough and fever), which showed amplification with up to 131,600 copies/mL. Her EBV viral load has been consistent since then. She developed acute abdominal pain and an appendiceal perforation was diagnosed 8 years post-transplant. Two liver masses were incidentally identified in the left and right hepatic lobes by abdominal computed tomography (CT) scan. Both appeared hypoechoic and individually measured up to 2.5 cm. Whole body positron emission tomography (PET)-CT scan was performed thereafter with no evidence of lymphadenopathy or other lesions. The mass in the right lobe was excised and found to be well circumscribed, white, and firm on gross examination. The mass in the left lobe was not removed due to its deep location.
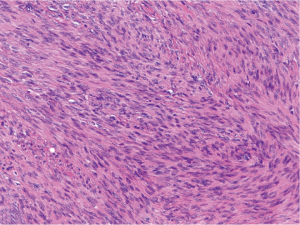
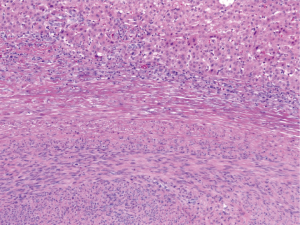
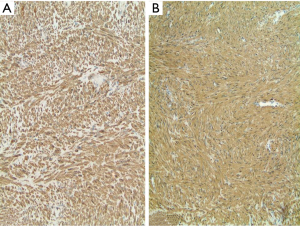
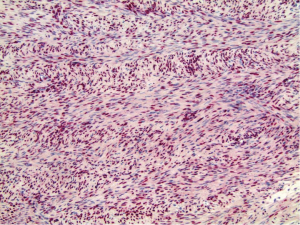
Histologically, the hepatic lesion was well demarcated from the adjacent liver parenchyma, and exhibited interlacing fascicles of spindle cells with eosinophilic cytoplasm and elongated, blunt-ended nuclei with stippled chromatin (Figure 1). Rare (less than 5%) primitive round cell component was identified. There was no evidence of necrosis or hemorrhage. Few small blood vessels were interspersed within the fascicles of spindle cells. Mitotic activity was less than 1 per 10 high power fields (HPF). Occasional lymphoid cells were seen at the junction between the tumor and adjacent liver parenchyma (Figure 2). Immunohistochemical (IHC) stains were performed to categorize the lesion. Smooth muscle actin (Figure 3A) and HHF 35 (Figure 3B) were positive, and desmin showed focal and weak equivocal staining consistent with smooth muscle origin. Ki67 showed only 1–2% of proliferative activity. EBV in situ hybridization was positive with a diffuse staining pattern (Figure 4), diagnostic of EBV infection. All these features were consistent with a diagnosis of EBV-SMT.
Whole body PET-CT scan performed 4 days after surgery showed a new intramuscular lesion in left anterior abdominal wall and a new left upper quadrant mesenteric metastatic mass (corresponding with an ill-defined 1.1 cm soft tissue density). Her EBV viral load 3 days after surgery was 60,400 copies/mL by PCR. Immediately prior to surgery, the EBV viral load had been 16,000 copies/mL. Subsequent to the pathologic diagnosis, immunosuppression was switched from tacrolimus to the mechanistic target of rapamycin (mTOR) inhibitor, everolimus. Her EBV viral load after 2 months of therapy dropped to 39,900 copies/mL.
Discussion
Post-transplant SMTs are a rare entity first described by Pritzker et al. in 1970 (7). Twenty-five years later, their association with EBV was described by Lee et al. (8). Although EBV-SMTs can be seen in different clinical settings in immunosuppressed patient, the majority of patients affected by EBV-SMT are (60%) posttransplant (9) and in HIV patients (10). Few cases associated with congenital immunodeficiency are also reported.
The cells of origin for EBV-SMT are thought to be derived from aberrant myogenous venous wall cells (6). As smooth muscle cells are not an usual target for EBV infection, immunosuppression may allow an abnormal entry of EBV into smooth muscle cells which could lead to a latent infection and subsequent neoplastic transformation altered by cytogenetic events (11). The underlying pathogenesis of EBV-related lymphomas has been well established, and found to be due to latent infection of B-lymphocytes through CD21 receptors with subsequent neoplastic proliferation. A similar mechanism was proposed for EBV-SMT, except CD21 receptors are not detected on smooth muscle cells. Fusion of smooth muscle cells with an infected B-lymphocyte prior to tumor proliferation has therefore been postulated (12). EBV has two strains (types 1 and 2) with different biological behavior, which are distinguished by polymorphisms in EBNA genes. Type 1 was identified in immunocompetent patients, and type 2 was detected in EBV-SMT (6). The EBV genome has more than 100 genes, but only a few are relevant in transmission and replication, including the latent membrane proteins (LMP1, LMP2A, and LMP2b), EBV nuclear antigens (EBNA1, EBNA2, EBNA3A, EBNA3B and EBNA3C), and EBV early RNAs (EBER1, and EBER2).
Overexpression of myc was observed in some of the EBV-SMT, which results in cellular hyperplasia (13). The latent membrane proteins (LMP2A) of EBV have been shown to activate mTOR (14). Activation of AKT/mTOR pathway was demonstrated in post transplantation related SMTs (15).
EBV-SMTs in pediatric patients appear earlier (interval between transplantation and tumor: mean, 42 months; range, 1.5–84 months) compared with adult patients (mean, 73–114 months) (14,16,17), and have a higher mortality rate. It has been hypothesized that the greater risk of EBV infection in children is related to the shorter interval from transplantation to the diagnosis of EBV-SMT. The lack of prior EBV immunity may additionally contribute to the higher mortality rate in children (18). A review by Jossen et al. found a female predominance of EBV-SMTs following solid organ transplantation (21 female patients and only 8 male patients) (19). Most EBV-SMTs are multifocal at the time of diagnosis, with some occurring in sites with little smooth muscle, such as the dura, spleen, tonsils, and glottis (6,20). Their multifocal nature is thought to be the result of multiple independent infections, rather than metastasis (6). Despite the multifocal nature of the disease, EBV-SMTs mostly appear to be indolent and discovered by means of surveillance imaging studies, especially in the absence of intracranial involvement (8,21). However, fever, abdominal pain, anorexia, weight loss, graft dysfunction, neurologic symptoms and pulmonary symptoms has been reported (8,22-24). Our patient's liver lesions were discovered subsequent to work up for an appendiceal perforation.
Histologically, EBV-SMTs are typically well-differentiated SMTs that show mild nuclear pleomorphism with no or inconspicuous mitotic activity, and frequent round cells and prominent intratumoral T lymphocytes (6). Three histologic classifications are used to describe the EBV-SMTs: leiomyoma, tumor of uncertain malignant potential, and leiomyosarcoma (9). However, morphologic features have not been shown to predict the clinical and biological behaviors of EBV-SMTs, as some well-differentiated tumors are lethal (8), while others classified as leiomyosarcomas follow a more benign clinical course (9,21,25,26). The location and immune status of the patient appear to play a more significant role in the clinical outcome. Therefore, it was proposed that all EBV-SMTs be designated as having uncertain malignant potential. EBV involvement using in situ hybridization for EBV-encoded small RNA (EBER) confirmed the diagnosis of EBV-SMT in all reports. Association with PTLD is common, ranging from 38–82% (18,19). Although PTLD was the working diagnosis at the time of appendiceal surgery, to date no PTLD has been identified in our patient.
The treatments for EBV-SMT in post-transplantation patients reported in the literature include surgical resection, reduction in immunosuppression, anti-viral therapies, and chemotherapy (27-29). Given the multifocal nature of the majority of these tumors, surgical resection can be challenging. However, multiple case reports suggested this approach as a first line treatment if surgical resection was possible (8,21). Reduction in immunosuppression to induce an immune response to the viral antigens is a commonly used approach. Sirolimus, a mTOR inhibitor, has shown efficacy in tumors with mTOR pathway activations (30). In addition, sirolimus also appears to prevent oncogenesis (31). In EBV-SMT, it is suggested that the AKT/mTOR pathway is activated and that inhibition of this pathway may have therapeutic benefits (14,32). Reports have described spontaneous tumor regression after conversion to sirolimus compared to those who remained on cyclosporine (17). In our patient, the right liver lobe lesion was redemonstrated and there was no significant reduction in size 3 months after starting treatment.
In conclusion, EBV-SMTs are rare tumors in pediatric patients, but should remain in the differential diagnosis in post-transplantation patients. The multifocal nature of these tumors and concurrence with PTLD should prompt evaluation for additional lesions after the diagnosis of EBV-SMT, as discovery of additional tumors may alter the treatment approach. Sirolimus, or a comparable mTOR inhibitor, in combination with surgical resection appears to be effective in the treatment of post-transplantation patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Cohen JI, Bollard CM, Khanna R, et al. Current understanding of the role of Epstein-Barr virus in lymphomagenesis and therapeutic approaches to EBV-associated lymphomas. Leuk Lymphoma 2008;49 Suppl 1:27-34. [Crossref] [PubMed]
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 2004;350:1328-37. [Crossref] [PubMed]
- Schulz TF. Cancer and viral infections in immunocompromised individuals. Int J Cancer 2009;125:1755-63. [Crossref] [PubMed]
- Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59-67. [Crossref] [PubMed]
- Suankratay C, Shuangshoti S, Mutirangura A, et al. Epstein-Barr virus infection-associated smooth-muscle tumors in patients with AIDS. Clin Infect Dis 2005;40:1521-8. [Crossref] [PubMed]
- Deyrup AT, Lee VK, Hill CE, et al. Epstein-Barr virus-associated smooth muscle tumors are distinctive mesenchymal tumors reflecting multiple infection events: a clinicopathologic and molecular analysis of 29 tumors from 19 patients. Am J Surg Pathol 2006;30:75-82. [Crossref] [PubMed]
- Pritzker KP, Huang SN, Marshall KG. Malignant tumours following immunosuppressive therapy. Can Med Assoc J 1970;103:1362-5. [PubMed]
- Lee ES, Locker J, Nalesnik M, et al. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N Engl J Med 1995;332:19-25. [Crossref] [PubMed]
- Kazmi SA, Aizenberg MR, Harper JL, et al. Multifocal histologically malignant Epstein-Barr virus-associated smooth muscle tumor in a pediatric transplant patient with an indolent course. Int J Surg Pathol 2014;22:186-9. [Crossref] [PubMed]
- Dekate J, Chetty R. Epstein-barr virus-associated smooth muscle tumor. Arch Pathol Lab Med 2016;140:718-22. [Crossref] [PubMed]
- Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol 2006;1:375-404. [Crossref] [PubMed]
- Purgina B, Rao UN, Miettinen M, et al. AIDS-related EBV-associated smooth muscle tumors: a review of 64 published cases. Patholog Res Int 2011;2011:561548. [Crossref] [PubMed]
- Jenson HB, Montalvo EA, McClain KL, et al. Characterization of natural Epstein-Barr virus infection and replication in smooth muscle cells from a leiomyosarcoma. J Med Virol 1999;57:36-46. [Crossref] [PubMed]
- Ong KW, Teo M, Lee V, et al. Expression of EBV latent antigens, mammalian target of rapamycin, and tumor suppression genes in EBV-positive smooth muscle tumors: clinical and therapeutic implications. Clin Cancer Res 2009;15:5350-8. [Crossref] [PubMed]
- Shen Q, Feng W, Long MS, et al. Multicentric hepatic EBV-associated smooth muscle tumors in an AIDS patient: a case report, investigation of mTOR activation and review of the literature. Int J Clin Exp Pathol 2011;4:421-9. [PubMed]
- Jonigk D, Laenger F, Maegel L, et al. Molecular and clinicopathological analysis of Epstein-Barr virus-associated posttransplant smooth muscle tumors. Am J Transplant 2012;12:1908-17. [Crossref] [PubMed]
- Tan CS, Loh HL, Foo MW, et al. Epstein-Barr virus-associated smooth muscle tumors after kidney transplantation: treatment and outcomes in a single center. Clin Transplant 2013;27:E462-8. [Crossref] [PubMed]
- Collins MH, Montone KT, Leahey AM, et al. Metachronous Epstein-Barr virus-related smooth muscle tumors in a child after heart transplantation: case report and review of the literature. J Pediatr Surg 2001;36:1452-5. [Crossref] [PubMed]
- Jossen J, Chu J, Hotchkiss H, et al. Epstein-Barr virus-associated smooth muscle tumors in children following solid organ transplantation: a review. Pediatr Transplant 2015;19:235-43. [Crossref] [PubMed]
- Suwansirikul S, Sukpan K, Sittitrai P, et al. Epstein-Barr virus-associated smooth muscle tumor of the tonsil. Auris Nasus Larynx 2012;39:329-32. [Crossref] [PubMed]
- Timmons CF, Dawson DB, Richards CS, et al. Epstein-Barr virus-associated leiomyosarcomas in liver transplantation recipients. Origin from either donor or recipient tissue. Cancer 1995;76:1481-9. [Crossref] [PubMed]
- Ha C, Haller JO, Rollins NK. Smooth muscle tumors in immunocompromised (HIV negative) children. Pediatr Radiol 1993;23:413-4. [Crossref] [PubMed]
- Davidoff AM, Hebra A, Clark BJ 3rd, et al. Epstein-Barr virus-associated hepatic smooth muscle neoplasm in a cardiac transplant recipient. Transplantation 1996;61:515-7. [Crossref] [PubMed]
- Chadwick EG, Connor EJ, Hanson IC, et al. Tumors of smooth-muscle origin in HIV-infected children. JAMA 1990;263:3182-4. [Crossref] [PubMed]
- Brichard B, Smets F, Sokal E, et al. Unusual evolution of an Epstein–Barrvirus-associated leiomyosarcoma occurringafter liver transplantation. Pediatr Transplant 2001;5:365-9. [Crossref] [PubMed]
- Suzuki K, Urushihara N, Fukumoto K, et al. A case of Epstein-Barr virus-associated pulmonary leiomyosarcoma arising five yr after a pediatric renal transplant. Pediatr Transplant 2011;15:E145-8. [Crossref] [PubMed]
- Sprangers B, Smets S, Sagaert X, et al. Posttransplant Epstein-Barr virus-associated myogenic tumors: case report and review of the literature. Am J Transplant 2008;8:253-8. [Crossref] [PubMed]
- Kingma DW, Shad A, Tsokos M, et al. Epstein-Barr virus (EBV)-associated smooth-muscle tumor arising in a post-transplant patient treated successfully for two PT-EBV-associated large-cell lymphomas. Case report. Am J Surg Pathol 1996;20:1511-9. [Crossref] [PubMed]
- Bonatti H, Hoefer D, Rogatsch H, et al. Successful management of recurrent Epstein-Barr virus-associated multilocular leiomyosarcoma after cardiac transplantation. Transplant Proc 2005;37:1839-44. [Crossref] [PubMed]
- Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol 2010;7:209-19. [Crossref] [PubMed]
- Blagosklonny MV. Immunosuppressants in cancer prevention and therapy. Oncoimmunology 2013;2:e26961. [Crossref] [PubMed]
- Toh HC, Teo M, Ong KW, et al. Use of sirolimus for Epstein-Barr virus-positive smooth-muscle tumour. Lancet Oncol 2006;7:955-7. [Crossref] [PubMed]


