Status of targeted therapies in the adjuvant treatment of colon cancer
Overview of adjuvant therapy in colon cancer
With roughly 100,000 cases diagnosed yearly, colon cancer is the 4th most frequently diagnosed cancer and the 2nd leading cause of cancer related death in the United States (1). Roughly 80% of cases are Stage I-III thus potentially curable with either surgery alone or combination therapy with surgery and chemotherapy (2). The 5-year overall survival (OS) for patients with Stage III colon cancer is 60%, however this decreases to 5% in patients with metastatic disease, underscoring the importance of adjuvant chemotherapy to abolish micrometastatic disease present at the time of curative surgical excision.
Multiple randomized controlled trials have established the benefit of 5-fluorouracil (5-FU) (3-5) and capecitabine (6) based regimens in the adjuvant setting for high-risk Stage II and Stage III colon cancer. 5-FU based adjuvant therapy results in a 5% OS benefit and 10% OS benefit in Stage II and Stage III patients, respectively, at 8 years (4). Two large, multinational, randomized studies [MOSIAC (7) and NSABP C-07 (8)] showed that adding oxaliplatin to 5-FU/leucovorin improves disease free survival (DFS) for Stage II/III colon cancer with decreases in relative recurrence risk by 20-23% (7-9). An updated subset analysis of the MOSAIC trial, however, questioned the benefit of the addition of oxaliplatin in low risk stage II patients and the elderly (70-75 years of age) (10). Based on available data, the NCCN guidelines currently recommend adjuvant 5-FU +/- oxaliplatin containing regimens for resected high risk Stage II and all Stage III colon adenocarcinoma (11). According to the NCCN, high risk Stage II disease is defined as tumors with poorly differentiated histology (in absence of microsatellite instability), lymphatic, vascular or perineural invasion, bowel obstruction or bowel perforation, close or positive resection margins, or less than 12 lymph nodes examined.
Many questions exist, however, regarding the absolute benefit of adjuvant chemotherapy. For example, most of the adjuvant trials included both Stage II and Stage III patients. Even when patients were sub-divided by stage at time of analysis, data for sub-stages such as Stage IIIA, IIIB and IIIC are pooled. Conceivably, there are differences in the benefit of adjuvant chemotherapy between patient sub-groups however the trials are not powered to detect these subtleties. Differences in adjuvant benefit likely also exist based on gene expression profiling however, to date, predictive benefits of therapy for a defined high risk group beyond the NCCN criteria for high risk Stage II and Stage III disease have not been established.
Interestingly, not all chemotherapeutic agents have shown benefit in the adjuvant setting. For example, despite proven benefit in metastatic colon cancer (12), irinotecan has not shown benefit in the adjuvant setting. While signals of activity were seen in one trial, overall there were no statistically significant differences in DFS or OS with the addition of irinotecan to 5-FU/leucovorin in the adjuvant setting (13-15). This finding gave an early indication that the mechanism of chemotherapy action might be different in the setting of macrometastatic versus micrometastatic disease, a theme that has pervaded the testing of biologic agents in adjuvant colon cancer as well.
Biologic agents in colon cancer
Anti-VEGF therapy
Vascular endothelial growth factor (VEGF) regulates angiogenesis both in health and disease, and contributes to angiogenesis in malignancy (16). For this reason, bevacizumab (Avastin®), a humanized monoclonal antibody to circulating vascular endothelial growth factor A (VEGF-A) was developed. Preclinical studies have shown multiple mechanisms of action for bevacizumab including inhibition of angiogenesis (17) by pruning of existing vessels and normalization of aberrant vessels which is thought to improve delivery of concurrently administered chemotherapy (18). Notably, however, bevacizumab is thought to be cytostatic rather than cytotoxic, which may explain its success only in combination with cytotoxic chemotherapy, rather than as monotherapy (17). Of note, however, the majority of pre-clinical work with bevacizumab has been in models of metastatic disease and the importance of these mechanisms of action are less clear in the adjuvant setting.
Clinically, in 2004, bevacizumab received Food and Drug Administration (FDA) approval for use as first line therapy in metastatic colorectal cancer based on studies showing improved response rate (RR), progression free survival (PFS), and OS when bevacizumab was added to 5-FU containing regimens (19). Soon thereafter, approval for use in the 2nd line metastatic setting was granted, again based on studies indicating improved OS in combination with 5-FU containing regimens (20). In 2013, bevacizumab received an additional indication for continuation therapy at progression of metastatic disease based on data showing improved OS with ongoing bevacizumab use after progression when the chemotherapy backbone was changed (21).
In 2012, two additional anti-VEGF agents received FDA approval for use in metastatic colorectal cancer. Ziv-aflibercept (Zaltrap®) is a recombinant fusion protein with VEGF binding regions that function as decoy receptors binding intra- and extra-vascular VEGF-A such that they cannot bind to their usual receptors. The VELOUR trial showed improved OS with FOLIFRI plus ziv-aflibercept versus FOLFIFI plus placebo in metastatic colorectal cancer that progressed following an oxaliplatin-containing regimen (22). Regorafenib (Stivarga®) is an oral tyrosine kinase inhibitor that inhibits VEGF receptors 1 and 3. It is approved for use in the refractory, “last line” setting for patients who have progressed through multiple lines of chemotherapy, based on a modest OS improvement in the CORRECT trial.
Neither of these agents has been tested in the adjuvant setting (23).
Anti-EGFR therapy
The epidermal growth factor receptor (EGFR) regulates signaling pathways involved in cell differentiation, cell proliferation and angiogenesis. Cetuximab (Erbitux®) is a recombinant chimeric human murine immunoglobulin antibody that binds to and inhibits EGFR. A similar drug, panitumumab (Vectibix®), is a fully human monoclonal antibody that inhibits EGFR. By inhibiting EGFR, cetuximab and panitumumab act via multiple mechanisms including G1 cell cycle arrest, induction of apoptosis, inhibition of tumor angiogenesis and activated antibody-dependent cellular toxicity (24). Importantly, the anti-EGFR agents have shown clinical success only in tumors that are KRAS wild type, and not in those with KRAS activating mutations, as these mutations cause constitutive activation of signaling cascades downstream to EGFR (25). Therefore, KRAS mutation status is routinely tested prior to initiation of anti-EGFR therapy. Similarly, the anti-EGFR agents are most effective in tumors that are BRAF wild type (25,26).
Clinically, cetuximab has shown mixed results, with only some trials showing PFS and OS benefit. For example the CRYSTAL trial showed improved PFS with the addition of cetuximab to FOLFIRI in the first line metastatic setting in KRAS wild type patients (27). The PRIME study, an analogous trial with FOLFOX4 with or without panitumumab, also showed improvement in PFS of 1.6 months in the panitumumab group (28).
However, there have been large randomized trials including COIN (29) and NORDIC VII (30) that have shown no benefit with the addition of cetuximab to chemotherapy in the metastatic setting. Reasons postulated for the lack of benefit seen in these trials include reductions of chemotherapy doses (29) or duration of chemotherapy (30) in the cetuximab groups. Interestingly, sub-group analysis of both trials showed that lack of benefit with the addition of cetuximab was limited to patients receiving either capecitabine or bolus-FU, compared to those receiving infusional 5-FU. The question remains whether one chemotherapy backbone, namely FOLFOX versus FOLFIRI, is more effective in combination with targeted agents. The ongoing Intergroup C80405 trial hopes to answer this question by combining either cetuximab or bevacizumab with physician’s choice of chemotherapy backbone- either FOLFOX or FOLFIRI may be chosen. The results of this trial are eagerly awaited.
Cetuximab is FDA approved for use in KRAS wild type tumors in combination with chemotherapy for metastatic disease in both the first and second line settings. It is also approved as monotherapy after failure of both irinotecan- and oxaliplatin containing regimens. Panitumumab has similar indications, and is primarily used in patients intolerant to cetuximab due to hypersensitivity reactions.
Biologics in the adjuvant setting
Given the success of the addition of biologic agents to chemotherapy in the metastatic setting, multiple studies were attempted to investigate possible benefit of these agents in the adjuvant setting. Success of the anti-VEGF and anti-EGFR agents in the adjuvant setting was thought by some to be a foregone conclusion, looking to the adjuvant use of 5-FU and oxaliplatin as historical examples. However, it is important to note that drugs with clinical success in the metastatic setting do not always show success adjuvantly, with irinotecan being a key example of a surprise failure in the adjuvant setting (13-15).
Adjuvant bevacizumab
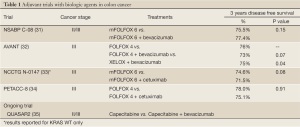
Two large randomized phase 3 trials investigated the use of bevacizumab with FOLFOX in the adjuvant setting. The NSABP (National Surgical Adjuvant Breast and Bowel Project) C-08 trial included 2,672 patients with resected stage II and III colon cancer (31). The standard therapy arm received mFOLFOX6 for a planned 12 cycles, and the experimental arm received the same with the addition of bevacizumab 5 mg/kg every two weeks for a year. Overall, this was a negative study. At a median follow up of 3 years, the DFS was 75.5% for the standard arm and 77.4% for the bevacizumab arm [hazard ration (HR) 0.89, 95% confidence interval (CI), 0.76-1.04, P=0.15]. Exploratory analysis found that there was a DFS benefit in favor of the bevacizumab group prior to 15 months of follow-up (HR 0.61; 95% CI, 0.48-0.78, P<0.0001), however this effect disappeared with longer follow up.
The AVANT (bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer) trial (32) was a multi-center, international trial that randomized 2,867 patients with resected stage III colon cancer to mFOLFOX4 for a planned 12 cycles versus mFOLFOX4 with bevacizumab 5 mg/kg every 2 weeks for 12 cycles followed by bevacizumab 7.5 mg/kg every 3 weeks for 8 additional cycles versus XELOX with bevacizumab 7.5 mg/kg every 3 weeks for 8 cycles followed by 8 additional cycles of bevacizumab monotherapy. There was no significant difference in 3-year DFS or 5-year OS between the three groups. In fact, there were numerically more relapses and deaths due to disease progression in the two bevacizumab containing arms, though these differences did not reach statistical significance. Similar to the NSABP trial, there was a decreased risk of relapse in the bevacizumab groups in the first 12 months of follow-up, however an increase in later relapses resulted in no overall differences between the groups.
Much has been made of the indication of transient benefit in the bevacizumab groups in both the NSABP and the AVANT trials. Specifically, relapse risk was decreased by 39% in the first 15 months in NSABP C-08 and by 37% in the first 12 months in the AVANT trial. However, in both cases, excess relapses after bevacizumab was discontinued resulted in no differences in DFS with longer follow-up. One explanation is that existing metastasis, too small to be seen on imaging, were maintained at a small size while exposed to bevacizumab, however had rebound growth upon discontinuation of anti-VEGF therapy. Another possibility is ascertainment bias noting that neither of these trials was placebo controlled. Regardless, the long term follow-up data showing no difference in DFS in either trial and even the concerning trend toward worse outcomes with bevacizumab in the case of the AVANT trial, indicate that adjuvant bevacizumab therapy is not appropriate clinical care for patients at this time.
The completed and ongoing trials studying bevacizumab in the adjuvant treatment of colon cancer are summarized in Table 1. QUASAR2 is an ongoing phase III international trial comparing capecitabine with capecitabine plus bevacizumab for the adjuvant treatment of stage II and III colorectal cancer (35). The primary endpoint is 3-year DFS. The study has fully accrued and study completion is anticipated in July 2014.
Full Table
Adjuvant cetuximab
The United States National Cancer Institute Intergroup Study N1047 trial evaluated 2,686 patients with resected stage 3 colon cancer, randomized to either mFOLFOX6 for 12 cycles or mFOLFOX6 with cetuximab at the standard dosing of 400 mg/m2 on day 1 of cycle 1, then 250 mg/m2 on day 8 of cycle 1 and days 1 and 8 of subsequent cycles (33). The trial was halted at interim analysis when, at a median follow up of 28 months, no benefit was seen with the addition of cetuximab regardless of KRAS or BRAF status. The 3-year DFS was 74.6% in the mFOLFOX6 group versus 71.5% in the mFOLFOX6/cetuximab group in patients with wild-type KRAS. In sub-group analysis, those ages 70 or older actually had decreased 3-year OS with the addition of cetuximab (86.2% vs. 72.5%, P=0.03). No evaluated sub-group showed any benefit from the addition of cetuximab.
Of note, the patients in the cetuximab arm received fewer cycles and lower doses of chemotherapy compared to patient in the mFOLFOX6 arm. Specifically, fewer patients in the cetuximab group were able to complete at least 6 cycles of chemotherapy (80% vs. 89%, P<0.001) and fewer received all 12 cycles (67% vs. 79%, P<0.001), though dosage intensity in the cycles given were similar between the groups.
The Pan-European Trials in Alimentary Tract Cancer (PETACC8) trial was presented at the European Society for Medical Oncology (ESMO) 14th World Congress on Gastrointestinal Cancer in 2012 and similarly showed no benefit to adding cetuximab to chemotherapy in the adjuvant setting (34). This phase 3 trial of 2,559 resected stage III colon cancer patients compared FOLFOX4 alone to FOLFOX4 with cetuximab. The interim analysis of the 1,602 KRAS wild-type patients after 39.6 months follow up showed no significant difference in DFS between the groups (78% FOLFOX4 alone, 75% cetuximab). Sub group analysis and long-term interim analysis are planned in the next few years.
The completed and ongoing trials studying cetuximab in the adjuvant treatment of colon cancer are summarized in Table 1.
Pathophysiology of macrometastasis versus micrometastasis
So why the failure of two classes of biologic agents- anti-VEGF and anti-EGFR- in the adjuvant setting despite success in metastatic disease? One explanation may be the differing pathophysiology of macrometastatic versus micrometastatic disease. Different genes, pathways and molecules may be required for a cell to establish itself as a metastatic foci (micrometastatic disease) rather than flourish as a metastatic mass. Micrometastasis may simply have different molecular features than macrometastasis and thus respond differently to biologic agents (36,37). Some have proposed that micrometastasis may actually grow faster than macrometastasis (Gompertz’s principle) (38), making them more responsive to cytotoxic chemotherapy than to biologic therapies widely thought to be cytostatic (17).
The evolution of a tumor with malignant potential to a tumor that actualizes that potential by establishing metastatic foci is complicated. Certainly the ability to create a new blood supply for tumor growth - angiogenesis- is required. Also required is the ability to make the epithelial-mesenchymal transition (39). Cell-cell adherence must initially be reduced allowing migration and spread (40) but later cells must have an analogous mesenchymal to epithelial transition to re-gain cell-cell adherence to make a stable metastasis (41). EGFR is thought to have a significant role in the epithelial-mesenchymal transition of metastatic cells (42).
The failure of biologic agents in the adjuvant setting supports the theory that micrometastasis behave differently than clinically apparent foci of metastatic disease. One theory is that micrometastatic disease may develop early resistance mechanisms to anti-angiogenic therapy such as increased invasiveness (43) or upregulation of pro-angiogenic mechanisms (44). Others hypothesize that tumor cell dormancy develops in the presence of adjuvant therapy, with tumor re-growth occurring once the biologic and chemotherapeutic agents are no longer present (45). Thus the early benefit of anti-VEGF agents seen in some of the adjuvant trials is lost once bevacizumab is discontinued when cells that were quiescent start to proliferate again (46).
Some preclinical and animal model data raise concerns that anti-VEGF therapies may actual select for a more aggressive tumor type with enhanced angiogenic capabilities (43,44,46,47). For example, in a mouse lung cancer model, cells treated with anti-VEGF agents exhibited 50-60% regression of tumor vasculature, however returned to pre-treatment vascularization levels with 7 days of removal of the anti-VEGF receptor drug (48). Similarly, Paez-Ribes et al. report that in mouse models of both pancreatic neuroendocrine carcinoma and glioblastoma, VEGF inhibitors concurrently exhibit anti-tumor effects and promote heightened invasiveness of tumor with increased lymphatic and distant metastasis (43). Indeed, induction of tumor hypoxia and an inflammatory state caused by anti-angiogenic agents may promote malignancy (43,47). Other animal models, however, have shown that treatment and subsequent discontinuation of anti-VEGF therapy resulted in tumor re-growth at a slower rate than control-treated animals, speaking against a so-called rebound growth effect (18).
Despite the above theoretical concerns, no clinical studies have indicated that exposure to biologic agents select for more aggressive tumors or promote tumor invasiveness. For example, a study in patients with gliobastoma multiforme treated with the pan-VEGF receptor tyrosine kinase inhibitor, cediranib, showed no rebound angiogenesis when the drug with withheld (49). And in multiple large, randomized clinical trials with bevacizumab in multiple disease types including renal cell carcinoma (50), breast cancer (51), and lung cancer (52), there have been no indication of re-bound tumor effect after withdrawal of bevacizumab.
Conclusions
New chemotherapy drug development has traditionally started with testing agents in the refractory, advanced disease setting, followed by the first line metastatic setting with only drugs with success in advanced disease advancing to testing in the adjuvant setting. However the assumption that drugs successful in macrometastatic disease will also be effective in micrometastatic disease (adjuvant setting) is increasingly being questioned, particularly in the era of biologic agents. In colon cancer, the benefit of cytotoxic agents such as 5-FU, capecitabine, and oxaliplatin did indeed translate to the adjuvant setting for most patient sub-groups. However, irinotecan showed no benefit in the adjuvant setting and bevacizumab and cetuximab even had trends towards worse outcomes when used adjuvantly. The importance of large-scale clinical trials of drugs in the exact settings in which they will be used cannot be overstated. An interesting concern is the idea that agents unsuccessful in the metastatic setting may show efficacy in the adjuvant setting. However, acting on this possibility would involve changing the paradigm of how we currently move new drugs through clinical trials with no current examples of such a drug at present.
Where do we go from here in the adjuvant treatment of colon cancer and other malignancies in the biologic era? Perhaps new classes of biologic agents such as inhibitors of insulin growth factor, MEK, PI3kinase or BRAF may be more successful. Or perhaps anti-VEGF or anti-EGFR therapies have a role, but we have to identify the correct patient population, with predictive markers. While plasma levels of VEGF-A or VEGFR-1 or 2 were not predictive of bevacizumab benefit in the AVANT trial (32), other studies have shown early signals. For example, genetic variations in the VEGF receptor genes may predict clinical response to bevacizumab in breast cancer (53). Similarly, the vascular normalization index in glioblastoma multiforme may predict response to the anti-VEGF tyrosine kinase inhibitor, cediranib (54). As additional targeted therapies are developed, validated biologic predictive markers must be determined to ensure these drugs are used in the patient population in which they are most likely to succeed. Additionally, it is imperative to understand the micro- and macro-environments in which these drugs function, and the differences in these environments in the adjuvant and metastatic settings. Finally, questions of optimal chemotherapeutic backbone must be addressed. Until then, the biologic agents will retain their clear role only in the metastatic disease setting for colorectal cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- National Cancer Insitute SEaERS. Available online: http://seer.cancer.gov/faststats, on Februray 20,2013.
- Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995;345:939-44. [PubMed]
- Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872-7. [PubMed]
- Andre T, Colin P, Louvet C, et al. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a randomized trial. J Clin Oncol 2003;21:2896-903. [PubMed]
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704. [PubMed]
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51. [PubMed]
- Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. [PubMed]
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71. [PubMed]
- Tournigand C, André T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353-60. [PubMed]
- Benson A B-ST, Chan E, et al. NCCN Guidelines Version 3.2013. Available online: http://www.nccn.org, on February 20, 2013..
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell,Italia Meridionale. J Clin Oncol 2005;23:4866-75. [PubMed]
- Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol 2007;25:3456-61. [PubMed]
- Ychou M, Raoul JL, Douillard JY, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802). Ann Oncol 2009;20:674-80. [PubMed]
- Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117-25. [PubMed]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997;18:4-25. [PubMed]
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 2008;8:579-91. [PubMed]
- Bagri A, Berry L, Gunter B, et al. Effects of anti-VEGF treatment duration on tumor growth, tumor regrowth, and treatment efficacy. Clin Cancer Res 2010;16:3887-900. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44. [PubMed]
- Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013;14:29-37. [PubMed]
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499-506. [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [PubMed]
- Vincenzi B, Schiavon G, Silletta M, et al. The biological properties of cetuximab. Crit Rev Oncol Hematol 2008;68:93-106. [PubMed]
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12. [PubMed]
- Schuch G, Kobold S, Bokemeyer C. Evolving role of cetuximab in the treatment of colorectal cancer. Cancer Manag Res 2009;1:79-88. [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [PubMed]
- Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103-14. [PubMed]
- Tveit K, Guren T, Glimelius B, et al. Randomized phase III study of 5-fluorouracil/floinate/oxaliplatin given continusousely or intermittently with or without cetuximab, as first-line treatment of metastatic colorectal cancer. Ann Oncol 2012;21:viii9.
- Allegra CJ, Yothers G, O’Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol 2011;29:11-6. [PubMed]
- de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225-33. [PubMed]
- Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383-93. [PubMed]
- Taieb J, Mini E. Adjuvant FOLFOX4 with or without cetuximab in patients with resected stage III colon cancer: DFS and overall survival results and subgroup analysis of the PETACC8 Intergroup phase III trial. Ann Oncol 2012;23:abstr LBA4.
- Available online: http://www.octo-oxford.org.uk/alltrials/infollowup/q2.html, AoM, 2013. QUASAR 2: A multicentre international study of capecitabine +/- bevacizumab as adjuvant treatment of colorectal cancer.
- Iiizumi M, Liu W, Pai SK, et al. Drug development against metastasis-related genes and their pathways: a rationale for cancer therapy. Biochim Biophys Acta 2008;1786:87-104.
- Riethdorf S, Wikman H, Pantel K. Review: Biological relevance of disseminated tumor cells in cancer patients. Int J Cancer 2008;123:1991-2006. [PubMed]
- Norton L. Conceptual and practical implications of breast tissue geometry: toward a more effective, less toxic therapy. Oncologist 2005;10:370-81. [PubMed]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442-54. [PubMed]
- Natalwala A, Spychal R, Tselepis C. Epithelial-mesenchymal transition mediated tumourigenesis in the gastrointestinal tract. World J Gastroenterol 2008;14:3792-7. [PubMed]
- Hugo H, Ackland ML, Blick T, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol 2007;213:374-83. [PubMed]
- Shin SY, Rath O, Zebisch A, et al. Functional roles of multiple feedback loops in extracellular signal-regulated kinase and Wnt signaling pathways that regulate epithelial-mesenchymal transition. Cancer Res 2010;70:6715-24. [PubMed]
- Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009;15:220-31. [PubMed]
- Ebos JM, Lee CR, Kerbel RS. Tumor and host-mediated pathways of resistance and disease progression in response to antiangiogenic therapy. Clin Cancer Res 2009;15:5020-5. [PubMed]
- Folkman J, Watson K, Ingber D, et al. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989;339:58-61. [PubMed]
- Loges S, Mazzone M, Hohensinner P, et al. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell 2009;15:167-70. [PubMed]
- Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009;15:232-9. [PubMed]
- Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 2006;116:2610-21. [PubMed]
- di Tomaso E, Snuderl M, Kamoun WS, et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res 2011;71:19-28. [PubMed]
- Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 2010;28:2144-50. [PubMed]
- Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-76. [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 2010;21:1804-9. [PubMed]
- Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 2008;26:4672-8. [PubMed]
- Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 2009;69:5296-300. [PubMed]


