Prognostic influence of histopathological regression patterns in rectal adenocarcinoma receiving neoadjuvant therapy
Introduction
Neoadjuvant chemoradiation therapy (CRT) followed by surgical resection has become the standard therapy for patients with locally-advanced rectal adenocarcinoma (1). This therapy has improved the outcome of these patients (2), but not all the cases show a good response to it and some patients might even progress during CRT with the consequent risks of tumor dissemination. There is much interest in developing systems that can predict response to therapy and also that can guide the need of adjuvant therapy after CRT. One of the factors that has been widely analyzed is pathological response to therapy. There are many systems to grade response. Initially most systems included five categories (3), but subsequent analysis concluded that three-tiered systems could correlate better with prognosis (4). The American Joint Committee on Cancer/College of American Pathologists (AJCC/CAP) recommended in 2010 to use a three tiered-system that considers the relative proportion of fibrosis and viable tumor cells, as proposed by Ryan et al. (4,5). Many authors have analyzed the prognostic significance of the different pathologic response grading systems with varying results (6,7), but very few studies have compared the different response patterns found in the tumor. The aim of this study is to analyze the pathologic response in our series of 183 patients from two Spanish hospitals and to determine whether there are different patterns of response and their prognostic significance.
Methods
We have retrospectively reviewed the files from the Departments of Surgical Pathology of Hospital Fundación Jiménez Díaz and Clínico San Carlos, both located in Madrid (Spain) and retrieved all the patients receiving CRT for rectal adenocarcinoma.
All the patients had a histological confirmatory diagnosis of malignancy and underwent an ultrasonographic endoscopy and a magnetic resonance imaging (MRI) to confirm tumor stage prior to therapy. All the cases with locally-advanced tumors were offered neoadjuvant therapy as per protocol in both centers. Standard neoadjuvant therapy included chemotherapy mainly based in capecitabine (825 mg/m2; 77% of the cases) or 5-fluorouracil (225 mg/m2) and 45 Gy over the pelvis, both following a standarized protocol approved in our Institution. Not more than two months after NAT end all the patients were operated, after MRI reevaluation of response. As part of the routine handling of the surgical resection specimens, both hospitals follow the recommendations of the Norwegian group for rectal cancer management, which was designed for the best evaluation of the mesorectal envelope margin and to which the Spanish Society of Surgeons has adhered (8,9). This protocol dictates the total paraffin embedment of the tumor bed after margin inking by the pathologists. Only patients with R0 resection were included in the present study.
We have collected demographic data and also clinical and pathological data related to the tumor. Two pathologists have independently reviewed all the hematoxylin-stained slides from the surgical specimen and graded response according to Ryan’s criteria, as recommended by the AJCC/CAP guidelines and also to Becker’s and Dvorak’s criteria. In short, in the CAP grading system cases were considered complete response (grade 0) when no residual tumor cells were found in the tumor bed; grade 1 when small islands of tumor remained, but overgrown by fibrosis; grade 2 for cases with more tumor cells, but still much fibrosis; and grade 3 for cases with clear predominance of viable tumor cells, with little pathological signs of response to therapy. In this grading system there is not a specific category for cases with no response, as is the rule in five-tiered systems. Both pathologists were blinded to the outcome of the patients, which was measured both as disease free survival (DFS) (time from therapy to local recurrence) and overall survival (OS) (time from therapy to death of disease), the primary endpoints of the analysis. In cases of discordance in grading a third pathologist reviewed the slides to assign a definite grade. In cases with no residual tumor on the first slides, serial sections of the paraffin blocks are mandatory.
Immunohistochemistry is not routinely performed, and it is only advised for cases in which there are extensive mucin pools with cells that are not clearly epithelial, to establish differential diagnosis between tumor cells and macrophages.
The results were described with mean (standard deviation) or percentage, as necessary. The interobserver concordance rate for tumor regression grading was estimated with the kappa score. For the analysis of association between variables we employed either xi square test or Fisher’s exact test. The survival analysis was based on the comparison of the Kaplan Meier survival curves with the log rank test. Finally we adjusted a Cox’s multivariate model both for DFS and OS as outcome measures of this study. The significance was settled at a P value <0.05 as usual.
The present study has been evaluated and approved by the Ethical Committees of both participating hospitals (14/197 ETFG with subsequent amendment of the main investigator for MJFA in Hospital Clínico and PIC65/2015 for Fundación Jiménez Díaz). This study follows all the regulations for personal data protection and patients have given written consent for participation in it.
Results
In our series 18% of the patients showed grade 0 (complete response), 31.7% grade 1, 19.2% grade 2 and 31.1% grade 3 response. We first analysed the interobserver concordance rate for regression grading using the CAP scheme and found a good concordance (kappa=0.82; P=0.00).
Table 1 summarizes the demographic features of the groups according to response. As can be seen, patients with grade 3 response showed a significantly higher rate of vascular invasion, perineural infiltration and lymph node involvement. Besides, relapse and death due to disease were more frequent in patients with regression grades 2 or 3 than in patients with complete or grade 1 regression.
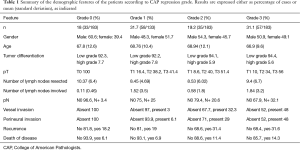
Full table
The main histopathologic pattern of regression found was fibrosis (93.4% of cases), either collagenous (29.2%) or cellular (64.2%). We found inflammatory reaction in 48.1% of cases and this inflammatory response was mainly eosinophilic in 37.7% of the cases. Necrosis was present in 47.2% of cases, either focal (35.8%) or diffuse (11.3%). We found mucin pools in 33% of the cases. The pattern of regression was tumor fragmentation in 42.5% of cases and tumor bulk reduction in 57.5%. The rate of vascular invasion and perineural infiltration was 21.7%.
Table 2 summarizes the histopathological features associated with regression. We found statistically significant associations between regression grade and the type of response (fragmentation vs. bulk reduction). The association between regression and fragmentation showed that most patients with bulk tumor reduction had regression grade 3 as opposed to those with fragmentation (31.1% vs. 13.3%). Besides, high grade tumors and necrosis were significantly more frequent among grade 3 cases.
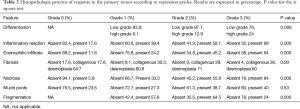
Full table
In a similar way, Table 3 summarizes the histopathological features associated with down-staging. We have found a statistically significant association between down-staging and regression grade (as expected), but also with vessel invasion, perineural infiltration and necrosis, which were significantly more frequent in patients that did not show down-staging. An interesting fact is that cases showing fragmentation showed significantly less down-staging than cases with bulk tumor reduction (37.8% vs. 59%).
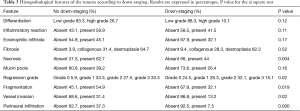
Full table
As for prognosis, the only factors predicting DFS in the univariate analysis were eosinophilic infiltrates, mucin pools, regression grade and T down-staging and the factors predicting OS were regression grade, mucin pools and perineural infiltration (but not down-staging). In Cox’s multivariate analysis of survival only T down-staging and mucin pools independently predicted DFS and regression grade and mucin pools predicted OS.
Discussion
Neoadjuvant therapy has become part of the standard management of patients with locally advanced rectal carcinoma in recent decades. CRT has been shown to significantly improve prognosis of these patients in many reports. However, there is still discussion regarding the need to employ adjuvant therapy, so there has been much interest in determining prognostic factors that influence outcome of patients after CRT. Many systems to grade regression have been proposed and validated in different studies, but in recent years it has become clear that three tiered systems are more reproducible and give the same prognostic information as five-tiered ones (3-5). The AJCC/CAP has recently proposed to use a three-tiered system based on Ryan et al. original work (4). A recent report by Mace et al. has definitively settled the prognostic influence of regression grade after CRT for rectal carcinoma in a large series of 538 patients (5). In this report, they have shown that regression is significantly associated to OS and also to local and distant recurrences. However, this study did not include down-staging in the multivariate analysis. T down-staging has been shown to be a more significant prognostic factor than regression in many reports so both factors should be analysed separately (9,10). In our series we have confirmed that both factors show prognostic influence in the univariate survival analysis but in the multivariate analysis down-staging showed independent prognostic value for DFS and regression for OS. According to our results we can conclude that down-staging is more related to local disease control, while regression is linked more to the risk of systemic relapse and cancer-specific death.
Another important issue is the morphological aspects considered in the regression grading schemes. Some regression grading systems (like Becker’s) consider the percentage of the tumor bed corresponding to viable tumor cells, but the system proposed by AJCC/CAP only considers the relative proportion of fibrosis and tumor. In this sense it is somehow subjective, for it does not mention any specific percentage (unlike other systems) and it does not take into account other possible patterns of response, like inflammation, necrosis or mucin pools. Few reports have analyzed the potential prognostic significance of the patterns of histopathological response to therapy (11,12) and they have centered in the difficulties these changes cause in evaluating stage, rather than in their potential prognostic significance.
It is fairly difficult to define whether the presence of mucin pools or a cellular fibrosis (desmoplasia) really represent response of the tumor to therapy or are already present in the primary tumor. It must be emphasized that in these cases we usually have only small endoscopic samples from the tumor and it is difficult to be sure of the histopathological features of the whole lesion prior to therapy. A recent report by Kim et al. (13) has shown that mucinous tumors are associated to a worse prognosis and less regression after therapy. Our study has reached similar conclusions for mucin pools in the tumor bed, either related to tumor itself or as a type of response to CRT, are significantly associated to a worse prognosis in terms of OS and DFS.
Mucin pools presence has been the only histopathological feature associated with prognosis. Neither the inflammatory reaction nor the kind of fibrosis have significantly influenced on the patients’ outcome. Two factors with well shown prognostic influence in previous reports, namely vascular invasion and perineural infiltration (14), have not behaved as independent significant prognosticators in the present study.
Another well-known fact is the frequent lack of concordance between MRI down-staging and regression grade. Clinicians are used to this discordance and some authors have proposed that the rate of tumor volume reduction can be more important than plain RECIST downsizing criteria for prognosis and propose dynamic MRI with volumetric measures as a more useful tool in this context (15,16). We feel that the explanation for this apparent discordance between MRI estimation of response and histopathological regression can be the pattern of regression. A recent report by Hav et al. (17) has evaluated this issue in a sample of 76 rectal carcinoma patients receiving CRT. These authors concluded that tumor shrinkage is prognostically different from tumor fragmentation and this can explain the worse predictive power of regression compared to down-staging. Our results in a larger series of 183 patients seem to confirm this supposition. We have shown that cases with down-staging usually show a bulk reduction pattern of response, while regression grade is usually worse in bulk reducing tumors. This is logical for if tumors reduce their size as a whole (bulk reduction) the relative proportion between fibrosis and tumor cells in the tumor bed will be necessarily higher than in cases with fragmentation, for in these cases the nests of tumor will be smaller. If we are to grade regression in the first case, it will probably fall into grade 2 or even 3, while the opposite will come true for fragmentation, as our results show.
Last there is no standarized recommendation for sample processing. In our hospitals, we follow the Norwegian protocol for rectal carcinoma designed to properly evaluate the state of the mesorectal envelope and which involves total embedment of the tumor bed. If the sampling is less exhaustive, we might overgrade regression, mainly in cases that show fragmentation (42.5% of our patients). These differences in sampling might explain at least in part the difference in the rates of regression between different studies.
In conclusion, we feel regression grade is essential as a prognostic tool in rectal carcinoma patients receiving NAT. However, if this factor is to be used for adjuvant therapy decision taking, it would be wise to establish a standarized protocol for sample management and also to incorporate into pathological reports the pattern of response (fragmentation vs. bulk reduction) and also some morphological data of response (mucin pools, inflammatory reaction). Our study is retrospective and prospective larger studies should include this factor in their analysis to further elucidate its real prognostic importance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethical Committees of both participating hospitals (14/197 ETFG with subsequent amendment of the main investigator for MJFA in Hospital Clínico and PIC65/2015 for Fundación Jiménez Díaz) and written informed consent was obtained from all patients.
References
- Benson AB 3rd, Bekaii-Saab T, Chan E, et al. Rectal cancer. J Natl Compr Canc Netw 2012;10:1528-64. [PubMed]
- Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol 2006;24:650-5. [Crossref] [PubMed]
- Losi L, Luppi G, Gavioli M, et al. Prognostic value of Dworak grade of regression (GR) in patients with rectal carcinoma treated with preoperative radiochemotherapy. Int J Colorectal Dis 2006;21:645-51. [Crossref] [PubMed]
- Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005;47:141-6. [Crossref] [PubMed]
- Mace AG, Pai RK, Stocchi L, et al. American Joint Committee on Cancer and College of American Pathologists regression grade: a new prognostic factor in rectal cancer. Dis Colon Rectum 2015;58:32-44. [Crossref] [PubMed]
- Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688-96. [Crossref] [PubMed]
- Abdul-Jalil KI, Sheehan KM, Kehoe J, et al. The prognostic value of tumour regression grade following neoadjuvant chemoradiation therapy for rectal cancer. Colorectal Dis 2014;16:O16-25. [Crossref] [PubMed]
- Wibe A, Syse A, Andersen E, et al. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum 2004;47:48-58. [Crossref] [PubMed]
- Ortiz H, Wibe A, Ciga MA, et al. Multicenter study of outcome in relation to the type of resection in rectal cancer. Dis Colon Rectum 2014;57:811-22. [Crossref] [PubMed]
- Fokas E, Liersch T, Fietkau R, et al. Downstage migration after neoadjuvant chemoradiotherapy for rectal cancer: the reverse of the Will Rogers phenomenon? Cancer 2015;121:1724-7. [Crossref] [PubMed]
- Shia J, Guillem JG, Moore HG, et al. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol 2004;28:215-23. [Crossref] [PubMed]
- Micev M, Micev-Cosić M, Todorović V, et al. Histopathology of residual rectal carcinoma following preoperative radiochemotherapy. Acta Chir Iugosl 2004;51:99-108. [Crossref] [PubMed]
- Kim TG, Park W, Choi DH, et al. Clinical significance of mucinous rectal adenocarcinoma following preoperative chemoradiotherapy and curative surgery. Tumori 2016;102:114-21. [Crossref] [PubMed]
- Cienfuegos JA, Rotellar F, Baixauli J, et al. Impact of perineural and lymphovascular invasion on oncological outcomes in rectal cancer treated with neoadjuvant chemoradiotherapy and surgery. Ann Surg Oncol 2015;22:916-23. [Crossref] [PubMed]
- Martens MH, van Heeswijk MM, van den Broek JJ, et al. Prospective, Multicenter Validation Study of Magnetic Resonance Volumetry for Response Assessment After Preoperative Chemoradiation in Rectal Cancer: Can the Results in the Literature be Reproduced? Int J Radiat Oncol Biol Phys 2015;93:1005-14. [Crossref] [PubMed]
- Xiao J, Tan Y, Li W, et al. Tumor volume reduction rate is superior to RECIST for predicting the pathological response of rectal cancer treated with neoadjuvant chemoradiation: Results from a prospective study. Oncol Lett 2015;9:2680-6. [PubMed]
- Hav M, Libbrecht L, Geboes K, et al. Prognostic value of tumor shrinkage versus fragmentation following radiochemotherapy and surgery for rectal cancer. Virchows Arch 2015;466:517-23. [Crossref] [PubMed]


