Clinical characteristics and treatment outcomes of patients with colorectal cancer who develop brain metastasis: a single institution experience
Introduction
About 10% of all cancer patients will develop brain metastasis (BM) during the course of their disease with incidence depending on the type of the primary tumor. BM is not a common event for patients with malignancies originating in the gastrointestinal tract, hepatobiliary system and pancreas (1). Specifically for colorectal cancer (CRC), the incidence of BM in retrospective studies is <5% of patients (1-6).
The typical patient with BM from CRC has a rectal primary and extra-cranial disease, particularly in the lung. These patients usually develop metachronous symptomatic disease in the brain late in the course of their disease with a median time from diagnosis of approximately 25 months (3-15). Metastatic disease to the brain in patients with CRC is associated with poor outcomes. In a SEER based study reviewing CRC cases from 2010–2011, the 1-year cause specific mortality was 29.6% vs. 90.0% for patients with and without BM respectively (3). Overall, median overall survival in retrospective series is reported to range from 2 up to 12 months (7,10-15). Outcomes reported in surgical series tend to be better, likely secondary to selection bias (1). Prognostic factors for overall survival after the diagnosis of BM are inconsistent in between different series but it appears that patients who are able to have surgical resection and additional systemic therapy have better survival (6,16,17). Prognostic score systems such as the recursive partitioning analysis (RPA) and diagnosis-specific graded prognostic assessment (DS-GPA) scores (18,19) are associated with outcomes (17,20).
In a retrospective review of patients with CRC and BM from Norway, the time interval between initial diagnosis and development of disease in the brain has increased over time, reflecting the progress made in developing better treatment strategies for control of systemic disease as well as better detection (21). Given the improvement in survival in metastatic CRC since the advent of novel biologics such as anti-vascular endothelial growth factor (VEGF) antibodies and anti-epithelial growth factor receptor (EGFR) antibodies, the expectation is that the incidence of BM would increase. However due to poor life expectancy and potential fear of intracranial hemorrhage from some novel biologics, patients with BMs have often been excluded from clinical trials assessing novel therapies. Therefore limited data on their clinical activity, clinical predictive markers to guide patient selection and safety of these agents exist in patients with BMs from CRC.
We sought to investigate the clinical characteristics, disease course and safety using biologics in our patients with CRC who develop BM.
Methods
Patients
This is a retrospective review of patients with CRC and BM treated at the Cancer Therapy and Research Center (CTRC) at the University of Texas Health Science Center at San Antonio (UTHSCSA). We included consecutive patients who presented for local treatment at the departments of Neurosurgery and Radiation Oncology between January 2005 and January 2015. ICD-9 codes 198.3, 198.4, 153, 154 were used to identify patients.
Data collection
Data collected included primary tumor location (colon/rectum), lymph node involvement, location of extra-cranial metastases, number and location of BMs, KRAS mutation status (wild-type vs. exon 2 mutation), BRAF mutation status, carcinoembryonic antigen (CEA) levels, presence of lymphovascular invasion (LVI) or perineural invasion (PNI), Eastern Cooperative Oncology Group (ECOG) performance status, RPA prognostic class I–III, treatment strategy for brain metastases [surgery, radiation therapy (RT), both, none], systemic treatment before and after the development of brain metastases (cytotoxic backbone and biological treatments).
The number and location of BM was determined by contrast-enhanced cross-sectional imaging of the brain (magnetic resonance imaging or computerized tomography scan). The RPA class was determined by the patients age, Karnofsky performance status (KPS) and presence of extra-cranial metastases as reported elsewhere (18). For patients who had more than one BM events, the first episode was recorded.
Treatments
Patients treated surgically had standard craniotomy. RT consisted of standard 3D conformal whole-brain radiotherapy and/or stereotactic radiosurgery (GammaKnife or Novalis system). Patients who received systemic therapy were treated with standard oxaliplatin- or irinotecan-fluoropyrimidine regimens with or without anti-VEGF or anti-EGFR antibodies according to national guidelines. All patients treated with an anti-VEGF antibody received bevacizumab.
Statistical analysis
Overall survival was calculated from the date of diagnosis of CRC to the date of death. Survival after diagnosis of metastatic disease was calculated from date of development of metastatic disease to the date of death. Survival after diagnosis of BMs was calculated from date of diagnosis of BMs to the date of death. The time to development of BM was calculated from the date of CRC diagnosis to the date of development of BM. Dates of diagnosis were considered the date with a first positive radiological finding or the day of pathological diagnosis, whichever occurred first. Survival curves were created using the Kaplan-Meier method and groups were compared using the log-rank test. The Fisher’s exact test was used to compare categorical variables. All calculations performed with R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Patients with missing data were excluded from analyses for the respective parameters.
Results
Patient characteristics
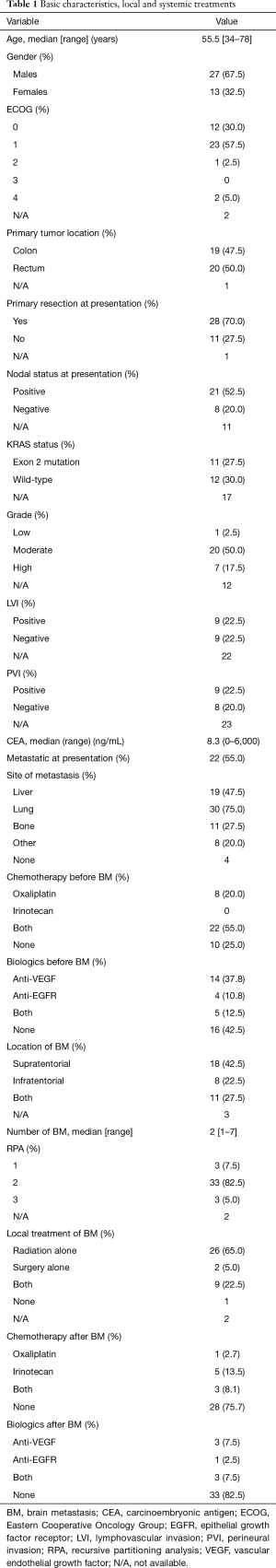
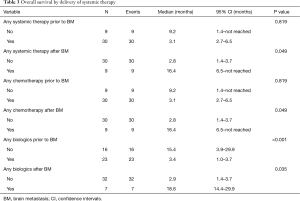
We identified 40 consecutive patients with CRC and BM using the Radiation Oncology and Neurosurgery databases. The basic characteristics at the time of diagnosis of CRC are presented in Table 1. Median age was 55.5 (range, 34–78) years, 67.5% were males, and 28% had a KRAS mutation. There were data on BRAF status only in one patient (wild-type). Seventy-five percent of the patients had lung metastasis at the time of BMs diagnosis. Five patients presented with synchronous brain metastases (12.5%). Two patients had no other evidence of extra-cranial disease (5%). One patient had leptomeningeal disease. Almost 25% of the patients were treatment-naive at the time of BMs diagnosis, 54% had received treatment with both an oxaliplatin- and irinotecan-based regimen. Close to 57% of the patients had received treatment with a monoclonal antibody, 8% had received both anti-VEGF and anti-EGFR directed therapy. Systemic therapy before and after the diagnosis of BM is shown in Table 1.
Full table
Disease characteristics and treatments
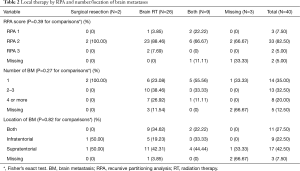
Characteristics of BMs and local treatment received are shown in Table 1. Patients had a median of 2 brain lesions (range, 1–7 brain lesions). Lesions were most frequently supratentorial (42.5%). In 27.5% of the patients lesions were both supra- and infra-tentorial. Most patients (90%) received brain RT, 65% as monotherapy while 22.5% of the patients had both surgical resection and brain RT. The type of local therapy by RPA and number/location of metastatic lesions is presented in Table 2. The type of local therapy was not significantly different based on RPA and number or location of metastatic lesions. Seventy-six percent of the patients did not receive any systemic therapy after BM diagnosis (Table 1). The most common regimen used after the diagnosis of BM was irinotecan-based. One patient did not receive any local (surgical resection or brain radiotherapy) therapy. This patient did not receive any systemic therapy as well and he passed 7 days after the diagnosis.
Full table
Treatment outcomes
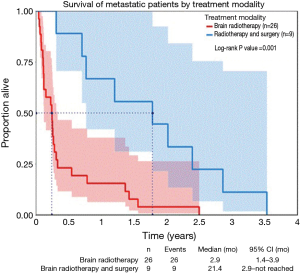
Median overall survival from time of primary tumor diagnosis was 36.4 months. Survival after diagnosis of any metastatic disease was 31.6 months, and 3.2 months after development of BM. Patients with KRAS wild-type tumors had longer overall survival compared to patients with KRAS mutated tumors (36.7 vs. 22.6 months, P=0.0263; HR, 0.36) but the survival after the diagnosis of BMs was not associated with KRAS status. RPA class I had the longest median overall survival (28.7 months), followed by class II (3.2 months). Patients with class III had the shortest overall survival (1.4 months). There was no overall survival difference with regards to primary tumor location, lymph node involvement, and CEA levels. Patients who received combined modality local therapy lived longer after the development of BMs compared to patients treated with surgical resection or radiotherapy alone (median survival 21.3 vs. 2.9 months, P=0.001, Figure 1). There was no difference in survival based on the location or the number of metastatic brain lesions.
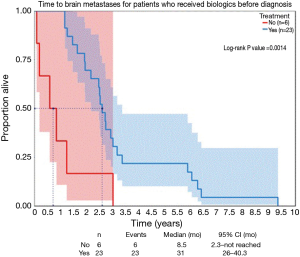
The time to development of BM for patients with metastatic disease treated with biologic agents was significantly longer compared to patients who had not received any biologics (Figure 2). Overall survival after the development of BM was not different based on prior exposure to cytotoxic chemotherapy, and was significantly better for the minority of patients who were able to receive cytotoxic systemic treatment after the development of BM (Table 3). The median survival after the diagnosis of BM for patients who received systemic therapy with or without biologics only prior to development of BM was 2.8 months. Patients who received treatment that incorporated biologics following development of BM had a median overall survival after BM diagnosis of 18.6 months (Table 3), it was 20 months for the patients who were not exposed to biologics before the diagnosis of BM. There were two patients who had chemotherapy without the addition of a biologic agent. Treatment with a biological agent after the diagnosis of BM was associated with improved survival while the opposite was true for exposure to biologics before the development of BM (Table 3). When patients exposed to anti-EGFR antibodies were excluded from analysis, exposure to biologics before development of BM was no longer associated with survival.
Full table
Safety
Overall, administration of biologic agents after development of BM was safe and well tolerated. There was only one case of intracranial hematoma formation at the resection cavity associated with recurrence at that area. This patient received only one bevacizumab infusion prior to this event. In general, for all six patients who received biologics after development of BMs, bevacizumab was subsequently added to the cytotoxic backbone after the first few cycles.
Discussion
Development of metastatic disease to the central nervous system is not a common event in patients with gastrointestinal malignancies and CRC specifically. It is usually occurs late in the course of disease and it is considered to harbor a grim prognosis. In our study, median overall survival was 3.2 months after the diagnosis of metastatic disease to the brain. Five (12.5%) of the patients had synchronous metastatic disease and two of them had no other evidence of metastatic extra-cranial disease. Even for the patients with synchronous disease in the brain outcomes are suboptimal, as only two patients survived more than 3 months and survival longer than 1 year was achieved in the single patient who was able to receive systemic therapy after local brain-directed therapy.
Disease presentation and outcomes are comparable to the ones reported in literature (6,7,11-13,15). In their surgical series, Mege and colleagues (11) report a frequency of synchronous BM of 43%, a finding likely related to selection bias.
The cumulative incidence of BM is higher in patients with RAS mutation harboring tumors (1.4% vs. 0.2% in all patients with CRC with a RAS mutation vs. wild-type and 14.5% vs. 2% after resection of hepatic metastases, hepatic artery infusion and systemic therapy for KRAS mutation vs. wild-type) (22,23). In a series from Memorial Sloan Kettering Cancer Center, RAS mutation was independently associated with the development of BM [HR, 3.7; 95% confidence intervals (CI), 1.7–8.1] (23). In this series, approximately 75% of the patients with BMs had tumors positive for a RAS mutation. In our patient population, 28% of the patients had a KRAS mutation, 31% were wild-type of KRAS and for the majority of patients (41%) the results for RAS mutation were not available. In our study, activating RAS mutation correlated with worse overall survival overall but not after the development of brain lesions.
In our patient population, combined modality local therapy (radiotherapy plus surgical resection) was associated with improved survival. Mege and colleagues report no benefit from administration of cranial radiotherapy after surgical resection. Most of the patients (80%) had as single brain lesion and none had more than two lesions (11). In the contrary, Damiens and colleagues report better survival for patients who were treated locally with combination of surgery and cranial radiotherapy (7). In their series, all patients who received combined modality therapy had a single brain lesion. Hammoud et al. reports as well better overall survival for patients treated with combined modality local therapy, even though the anatomical details for each treatment strategy (number and location of brain lesions) are not provided (8). In our case series, of the nine patients who were treated with combined modality local therapy, four had two or more brain lesions. Based on the global experience of improved outcomes with liver-directed therapies in metastatic CRC, it is likely that, for patients with BM, an aggressive local approach (maximal safe debulking and adjuvant cranial radiotherapy) is warranted as well.
As reported by other groups (6,17,24), administration of systemic therapy (cytotoxic and biological) after development of BM was associated with better survival. The details of the type of systemic therapy administered are not provided in the studies by Tokoro et al. and Nieder et al. Our study is the first to our knowledge to report improved outcomes specifically with administration of a biologic agent after the development of BM. The timing of administration of biologic agent therapy (only before or only after development of BM) was associated with survival, while this was not true for cytotoxic agent administration. Jung and colleagues as well as Baek and colleagues reported improved outcomes with less lines of therapy prior to development of BM (24,25). Unfortunately, the number of patients in our study who went on to receive systemic treatment was small, precluding any meaningful comparison between patients treated with oxaliplatin- vs. irinotecan-based chemotherapy and anti-VEGF vs. anti-EGFR antibodies. Patients who had already received treatment with biological agents before development of BM, had a significantly worse overall survival. When we included patients only exposed to anti-VEGF treatment, this difference was no longer significant. It appears that patients who have not excluded all available systemic treatment options are still able to have a survival at least comparable to patients who will never develop brain metastases.
Conclusions
In summary, BM is an uncommon and late event in the natural history of metastatic CRC. Treatment with biologic agents may delay the development of BM. The ability to deliver combined-modality local brain therapy as well as availability of more systemic therapy options appears to lead to improved outcomes.
Acknowledgements
C Fountzilas and K Chang are recipients of a Cancer Prevention and Research Institute of Texas (CPRIT) Research Training Award (RP140105).
Footnote
Conflicts of Interest: The results of this study were in part presented online only at the 2015 ASCO Annual Meeting.
Ethical Statement: This is a retrospective study. All patient info was de-identified. Chart review was performed by authors who were all staff at our institution. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- Lemke J, Scheele J, Kapapa T, et al. Brain metastases in gastrointestinal cancers: is there a role for surgery? Int J Mol Sci 2014;15:16816-30. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Qiu M, Hu J, Yang D, et al. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget 2015;6:38658-66. [PubMed]
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [Crossref] [PubMed]
- Sundermeyer ML, Meropol NJ, Rogatko A, et al. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer 2005;5:108-13. [Crossref] [PubMed]
- Tokoro T, Okuno K, Hida JC, et al. Prognostic factors for patients with advanced colorectal cancer and symptomatic brain metastases. Clin Colorectal Cancer 2014;13:226-31. [Crossref] [PubMed]
- Damiens K, Ayoub JP, Lemieux B, et al. Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Curr Oncol 2012;19:254-8. [Crossref] [PubMed]
- Hammoud MA, McCutcheon IE, Elsouki R, et al. Colorectal carcinoma and brain metastasis: distribution, treatment, and survival. Ann Surg Oncol 1996;3:453-63. [Crossref] [PubMed]
- Hugen N, van de Velde CJ, de Wilt JH, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 2014;25:651-7. [Crossref] [PubMed]
- Kruser TJ, Chao ST, Elson P, et al. Multidisciplinary management of colorectal brain metastases: a retrospective study. Cancer 2008;113:158-65. [Crossref] [PubMed]
- Mege D, Ouaissi M, Fuks D, et al. Patients with brain metastases from colorectal cancer are not condemned. Anticancer Res 2013;33:5645-8. [PubMed]
- Tan WS, Ho KS, Eu KW. Brain metastases in colorectal cancers. World J Surg 2009;33:817-21. [Crossref] [PubMed]
- Tanriverdi O, Kaytan-Saglam E, Ulger S, et al. The clinical and pathological features of 133 colorectal cancer patients with brain metastasis: a multicenter retrospective analysis of the Gastrointestinal Tumors Working Committee of the Turkish Oncology Group (TOG). Med Oncol 2014;31:152. [Crossref] [PubMed]
- Wroński M, Arbit E. Resection of brain metastases from colorectal carcinoma in 73 patients. Cancer 1999;85:1677-85. [Crossref] [PubMed]
- Michl M, Thurmaier J, Schubert-Fritschle G, et al. Brain Metastasis in Colorectal Cancer Patients: Survival and Analysis of Prognostic Factors. Clin Colorectal Cancer 2015;14:281-90. [Crossref] [PubMed]
- Kye BH, Kim HJ, Kang WK, et al. Brain metastases from colorectal cancer: the role of surgical resection in selected patients. Colorectal Dis 2012;14:e378-85. [Crossref] [PubMed]
- Nieder C, Hintz M, Grosu AL. Colorectal cancer metastatic to the brain: analysis of prognostic factors and impact of KRAS mutations on presentation and outcome. Clin Transl Oncol 2016;18:88-92. [Crossref] [PubMed]
- Gaspar LE, Scott C, Murray K, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys 2000;47:1001-6. [Crossref] [PubMed]
- Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010;77:655-61. [Crossref] [PubMed]
- Jiang XB, Yang QY, Sai K, et al. Brain metastases from colorectal carcinoma: a description of 60 cases in a single Chinese cancer center. Tumour Biol 2011;32:1249-56. [Crossref] [PubMed]
- Nieder C, Pawinski A, Balteskard L. Colorectal cancer metastatic to the brain: time trends in presentation and outcome. Oncology 2009;76:369-74. [Crossref] [PubMed]
- Kemeny NE, Chou JF, Capanu M, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer 2014;120:3965-71. [Crossref] [PubMed]
- Yaeger R, Cowell E, Chou JF, et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer 2015;121:1195-203. [Crossref] [PubMed]
- Baek JY, Kang MH, Hong YS, et al. Characteristics and prognosis of patients with colorectal cancer-associated brain metastases in the era of modern systemic chemotherapy. J Neurooncol 2011;104:745-53. [Crossref] [PubMed]
- Jung M, Ahn JB, Chang JH, et al. Brain metastases from colorectal carcinoma: prognostic factors and outcome. J Neurooncol 2011;101:49-55. [Crossref] [PubMed]


