Utility of the Edmonton Frail Scale in identifying frail elderly patients during treatment of colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the leading causes of death, and 70% of all cancer mortality occurs in patients that are 65 years and older (1). Elderly patients are at higher risk of complications from treatments, both due to impaired tolerability of chemotherapy and co-morbidities. Age has been associated with pharmacokinetic and pharmacodynamic changes that may increase susceptibility to toxicity (2). Clinical trials often fail to enroll representative numbers of elderly patients, in part due to the difficulties of these patients meeting strict eligibility criteria (3). Consequently, oncologists are often wary of treating elderly patients, and a number of studies have demonstrated lower rates of adjuvant therapy, and similar rates of chemotherapy toxicity (4,5). This suggests that well selected elderly patients can be effectively and safely treated similar to younger patients.
It is important that effective cancer treatment is not withheld, particularly if it is potentially curative, can prolong life, or palliate symptoms. Since there is significant heterogeneity in health status among elderly patients with CRC, chronological age alone is an insufficient measure of “fitness” for chemotherapy (6). In the geriatric literature, the concept of “frailty” has been postulated as a better indicator of “functional age” of the patients (7,8) and studies have suggested frailty to be a better predictor of mortality and treatment response than age (9). However, there is still considerable debate surrounding both its definition and the methods by which to measure it. One definition states frailty as ‘a biologic syndrome of decreased reserve and resistance to stressors, resulting from cumulative decline across multiple physiologic systems, and causing vulnerability to adverse outcomes’ (10).
Identifying factors that place patients with cancer at risk for frailty may help oncologists tailor treatment decisions to their individual patients (11,12). The comprehensive geriatric assessment (CGA) is considered by many to be the “gold standard” and is an effective clinical tool for identifying frailty in elderly patients (13,14). The CGA has demonstrated utility for the management of various geriatric problems including prevention of institutionalization and maintenance of independence, prevention of delirium in hospitalized patients, falls, and hospital readmission (15-17). It can also provide information about life expectancy and functional reserve as well as tolerance to and toxicity of chemotherapy (18,19). Although the CGA may provide additional information about “vulnerability” that clinical judgment and performance status do not, it may not be useful in routine clinical practice due to the time-consuming nature of its administration, and therefore simpler screening methods are required to identify frailty in routine clinical practice. One such screening instrument is the Edmonton Frail Scale (EFS); it requires less than five minutes to administer, and has been shown to be reliable, valid, and feasible for use by non-geriatricians (19). However, to date the EFS has not been evaluated in elderly cancer patients.
The primary objective of this study was to determine whether there was a correlation between frailty measured by the EFS and the likelihood of being offered chemotherapy. The secondary objectives of the study were to determine whether there was an association between EFS scores and: (I) chemotherapy modifications; (II) clinician’s impression of the patient’s functional status; and (III) hospitalizations resulting from chemotherapy toxicities.
Methods
This was a single-center, prospective, observational study conducted at the Juravinski Hospital and Cancer Center (JHCC), a tertiary care facility located in Hamilton, Ontario, Canada. Ethics approval for this project was obtained from the local Research and Ethics Board. Potential patients were identified as a convenience sample when they presented to the JHCC either as an initial consultation or for follow-up with a Medical Oncologist prior to starting therapy. All new CRC patients referred to the JHCC were screened by a study coordinator. Potentially eligible patients were identified prior to the consultation visit with the attending Medical Oncologist. If the Oncologist agreed to participate, then the patient was informed of the study and invited to consider participation. All patients provided informed consent prior to their involvement in the study.
Inclusion criteria included age 70 years and over, and histologically confirmed diagnosis of adenocarcinoma of the colon or rectum. Since the clinical manifestations, the likelihood of response to chemotherapy, and prognosis varies dramatically between cancer types, the study was purposefully restricted to patients with CRC. Patients were excluded from the study if they were not fluent in English or had difficulties with manual dexterity, blindness, or deafness, since the EFS has not been validated in such patients.
Prior to the patient’s appointment with the Medical Oncologist, the EFS was administered by the study coordinator, immediately scored, and the result placed in a sealed envelope. The resulting score from 0–17 categorizes patients as not frail [0–4], vulnerable [5–6], mild frailty [7–8], moderate frailty [9–10], and severe frailty (>11) (19). The treating oncology team, including the Nurse and the Medical Oncologist, and the patient were blinded to the EFS score. The EFS was re-administered one week later to test for reliability. The Medical Oncologist assessed the patient according to routine clinic practice. Following the consultation, the physician completed a study-specific summary form to provide details of the patient’s performance status, global health and treatment decision.
Since the time interval between clinic visits varies depending on the specific treatment chosen, the frequency of follow-up visits was left to the discretion of the treating physician. Medical chart reviews were performed every 2 months while on therapy by a study member not involved directly in the patients’ care; follow-up for adjuvant patients was to the end of therapy, and metastatic patients until the end of first-line therapy in order to assess hospitalizations attributed to treatment, and response to therapy. Information extracted from the charts included details about the administration of chemotherapy and reasons for not offering chemotherapy in applicable patients, chemotherapy dose modifications or regimen changes, major treatment-related side effects (febrile neutropenia, anemia or bleeding requiring blood transfusion, dehydration, diarrhea or nausea/vomiting requiring interventions other than those specified by standard chemotherapy protocols), hospitalizations, disease progression and death.
Statistical analysis
Baseline demographics were summarized using descriptive statistics. In order to ensure that treatment patterns of individual oncologists remained confidential, results were pooled and reported at the group level. Patients who completed chemotherapy were compared with those who did not. Reproducibility of the day 1 and day 7 EFS scores were examined using Pearson’s Correlation.
To determine whether EFS score was associated with physician’s clinical impression, the proportion of patients with an EFS scale ≥7 was assessed for five qualitative levels of fitness as determined by the treating medical oncologist: fitter, appropriately well, diminished reserves but coping, diminished reserves and having difficulty coping, and no reserves and in poor health. Multivariate analysis was used to determine the correlation between EFS scores and the oncologists’ treatment decision. Significance was set at a P value of 0.05. Statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
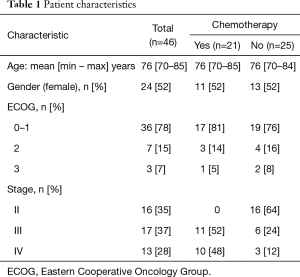
A total of 46 patients were recruited into the study. Approximately half of the recruited patients were female and the mean age was 76 years (range, 70–85 years). The characteristics of the study population are summarized in Table 1. The majority of patients had an Eastern Cooperative Oncology Group (ECOG) score of 0–1 (78%), and 35% were stage II, 37% were stage III and 28% were stage IV. Chemotherapy treatment was prescribed to just under half of the patients (n=21). There was no difference in the mean age, gender, or the performance status of the group of patients who received chemotherapy and those that did not.
Full table
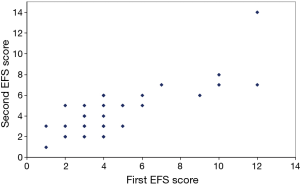
Thirty-two of the 46 patients completed a second EFS evaluation one week after the baseline evaluation to assess reproducibility. The mean EFS score for all patients was similar over the one-week period, and the EFS score showed high reproducibility between the baseline and week 1 administrations (Figure 1; r=0.81; 95% CI: 0.64–0.90, P<0.0001). Patients with reduced levels of fitness according to the physician’s clinical impression were significantly more likely to have EFS scores ≥7 (P<0.001; Table 2).

Full table
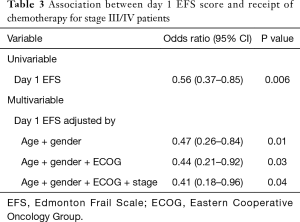
There was no correlation between the EFS and receipt of chemotherapy for the study population as a whole [odds ratio (OR): 0.89; 95% CI: 0.73–1.09, P=0.26]. Similarly, there was no correlation between ECOG score and receipt of chemotherapy for the study population as a whole (OR: 1.19; 95% CI: 0.23–6.11, P=0.83). As none of the 16 stage II patients were recommended chemotherapy (none had “high-risk features”), an exploratory analysis was conducted excluding these patients. There was a reduced likelihood of receiving chemotherapy for stage III/IV patients with higher EFS scores (Table 3; OR: 0.56; 95% CI: 0.37–0.85, P<0.01 per unit increment). In contrast, there was no correlation between ECOG scores and receipt of chemotherapy in this subset (OR: 2.27; 95% CI: 0.29–17.58, P=0.43). To determine whether this association was independent of the factors involved in the clinical decision-making process (age, gender, performance status and stage) a stepwise multivariable analysis was conducted. Higher EFS scores at day 1 remained predictive of lower odds of receiving chemotherapy, after adjustment for age, gender, performance status and tumour stage (OR: 0.41; 95% CI: 0.18–0.96, P<0.05).
Full table
To determine whether EFS scores had any predictive value for determining if changes were made to chemotherapy treatment (dose reduction and/or the physician switching to a single agent chemotherapy regime, e.g., combination chemotherapy to monotherapy), outcomes were compared between patients with low EFS scores (<7) and higher EFS scores (≥7). No difference was found between the two groups with respect to receiving an upfront dose reduction, monotherapy or either of these (all P>0.05, data not shown). Using the same cut points, EFS scores ≥7 were showed a trend towards higher rates of serious adverse events and hospitalizations (P=1.0 and P=0.01, respectively). EFS score was inversely related to overall survival (5.2 months for EFS ≥7, compared to 15.4 months for EFS <7, P=0.036; Table 4).

Full table
Conclusions
With an aging population, oncologists are increasingly assessing elderly patients for potential systemic therapies. Balancing the potential toxicity of treatment, quality-of-life concerns, and possible survival benefits in this population is paramount. At the present time, an oncologist’s ability to determine who is a frail patient prior to considering systemic therapy is limited. Chronological age on its own is a poor predictor of chemotherapy toxicity alone, as is assessment of performance status (12). An objective measure of frailty would be clinically beneficial to help differentiate between patients who would tolerate chemotherapy similar to the younger counterparts, and those patients that are too frail for chemotherapy or need closer observation to potentially avoid hospitalization.
We employed the EFS as it is an easy to administer scale that could be completed as part of routine practice. The current pilot study demonstrates a high degree of correlation between EFS scores and clinical judgment for assessment of frailty. The data from our exploratory study suggests that the EFS can identify patients that oncologists do not prescribe chemotherapy to, independent of performance status, age, gender, and stage. Although not our primary objective, it is interesting that higher EFS scores were associated with increased hospitalizations and worse overall survival in this small study. This scale has high inter-rater reliability, and potentially could provide useful information rapidly in clinical practice. It has been validated against the Geriatricians’ Clinical Impression of Frailty (GCIF) in a Canadian population referred for CGA (r=0.64) (19). The EFS samples ten domains and scores range from 0 (not frail) to 17 (maximal frailty) (19). Expert consensus and evidence suggests that an assessment tool like the EFS, that provides information about an elderly person’s “functional age” could potentially identify those who are more vulnerable to complications from cancer treatment (12). In a study comparing eight frailty scales (the Groningen Frailty Indicator, the Tilburg Frailty Indicator, a 70-item and a 44-item Frailty Index, the Clinical Frailty Scale, a frailty phenotype score, the Edmonton Frailty Scale, and the FRAIL scale), mortality was best predicted by the Frailty Index and Edmonton scales, with death rates three to five times higher in cases classified as frail compared to those not classified as frail (20).
Within geriatrics, the CGA has been viewed as the gold standard. The recent update from the International Society of Geriatric Oncology recommended the following domains be evaluated in the geriatric assessment: functional status, comorbidity, cognition, mental health status, fatigue, social status and support, nutrition, and presence of geriatric syndromes (12). However, it has been repeatedly recognized that major barriers to the routine use of the CGA including the length of a full assessment, and limited resources in geriatric oncology in many jurisdictions. In response to this, screening instruments have been developed. The Vulnerable Elder Survey (VES-13) did correlate with a CGA in a pilot study of 50 patients with metastatic prostate cancer, as well as more diverse population of hematological and solid malignancies (21,22). The G8 instrument has been developed by the ONCODAGE project in France, and there are comparative data against the VES-13 suggests better sensitivity (23). A recent systematic review found that both the VES-13, and G8 (along with other instruments) did not have sufficient discrimination ability to replace the CGA (24). In 2014, the International Society of Geriatric Oncology also was unable to endorse one tool over another (12). To date, the EFS has not been compared to these tools that have developed during a similar time period. We propose that a simpler and more time-efficient tool such as the EFS may help guide decisions about whether to administer chemotherapy to elderly patients, and potentially screen patients who would benefit from formal geriatric assessment.
There are a number of limitations of this study. We were unable to recruit patients who were not fluent in English or those with manual dexterity problems, blindness, or deafness because the EFS has not yet been validated in such patients. We can therefore not generalize the findings of our study across all elderly patients, however, this is likely a small percentage of all patients. In addition, fewer patients than anticipated received chemotherapy, partially due to the fact that we enrolled all stage 2–4 colorectal patients prior to a treatment decision, in an effort to reduce bias. Nonetheless, our exploratory analysis that excluded patients for whom chemotherapy was not felt to be necessary, suggests that the EFS could predict patients who were fit enough to receive chemotherapy. This pilot study requires confirmation in additional studies involving larger patient’s numbers. In addition, the EFS is not the gold-standard frailty assessment tool; and additional studies comparing the EFS to other oncology specific frailty assessment tools would be required before widespread implementation of this scale is considered. Additional strengths of our study include several measures utilized to reduce bias, including the blinding of the physicians, patients and nurses to the EFS scores, and using different investigators to administer the EFS and to undertake the chart review.
In summary, our study suggests that the EFS could provide a reproducible, quantifiable measure of frailty which, when coupled with clinical judgment, may enhance the decision-making process even further. Future work may examine whether utilization of the EFS can assist clinicians and patients in discussing treatment options, rehabilitation and unplanned health care utilization in elderly cancer patients compared to clinical judgment alone. It is pertinent to determine whether interventions to correct for frailty in elderly patients can improve tolerance to chemotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics approval for this project was obtained from the Hamilton Integrated Research Ethics Board (No. IRB00009502) and all patients provided informed consent prior to their involvement in the study.
References
- Yancik R, Ries LA. Aging and cancer in America. Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am 2000;14:17-23. [Crossref] [PubMed]
- Shi S, Mörike K, Klotz U. The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol 2008;64:183-99. [Crossref] [PubMed]
- Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 2005;23:3112-24. [Crossref] [PubMed]
- Ko JJ, Kennecke HF, Lim HJ, et al. Reasons for Underuse of Adjuvant Chemotherapy in Elderly Patients With Stage III Colon Cancer. Clin Colorectal Cancer 2016;15:179-85. [Crossref] [PubMed]
- Popescu RA, Norman A, Ross PJ, et al. Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol 1999;17:2412-8. [PubMed]
- Puts MT, Santos B, Hardt J, et al. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol 2014;25:307-15. [Crossref] [PubMed]
- Rockwood K, Fox RA, Stolee P, et al. Frailty in elderly people: an evolving concept. CMAJ 1994;150:489-95. [PubMed]
- Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol 2013;4:218-26. [Crossref] [PubMed]
- Rockwood K, Stadnyk K, MacKnight C, et al. A brief clinical instrument to classify frailty in elderly people. Lancet 1999;353:205-6. [Crossref] [PubMed]
- Rønning B, Wyller TB, Jordhøy MS, et al. Frailty indicators and functional status in older patients after colorectal cancer surgery. J Geriatr Oncol 2014;5:26-32. [Crossref] [PubMed]
- Puts MT, Hardt J, Monette J, et al. Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst 2012;104:1133-63. [Crossref] [PubMed]
- Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595-603. [Crossref] [PubMed]
- Reuben DB, Frank JC, Hirsch SH, et al. A randomized clinical trial of outpatient comprehensive geriatric assessment coupled with an intervention to increase adherence to recommendations. J Am Geriatr Soc 1999;47:269-76. [Crossref] [PubMed]
- Extermann M, Meyer J, McGinnis M, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol 2004;49:69-75. [Crossref] [PubMed]
- Giantin V, Valentini E, Iasevoli M, et al. Does the Multidimensional Prognostic Index (MPI), based on a Comprehensive Geriatric Assessment (CGA), predict mortality in cancer patients? Results of a prospective observational trial. J Geriatr Oncol 2013;4:208-17. [Crossref] [PubMed]
- Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med 1994;331:821-7. [Crossref] [PubMed]
- Extermann M, Aapro M. Assessment of the older cancer patient. Hematol Oncol Clin North Am 2000;14:63-77. viii-ix. [Crossref] [PubMed]
- Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol 2005;23:6865-72. [Crossref] [PubMed]
- Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006;35:526-9. [Crossref] [PubMed]
- Theou O, Brothers TD, Mitnitski A, et al. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013;61:1537-51. [Crossref] [PubMed]
- Luciani A, Ascione G, Bertuzzi C, et al. Detecting disabilities in older patients with cancer: comparison between comprehensive geriatric assessment and vulnerable elders survey-13. J Clin Oncol 2010;28:2046-50. [Crossref] [PubMed]
- Mohile SG, Bylow K, Dale W, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer 2007;109:802-10. [Crossref] [PubMed]
- Soubeyran P, Bellera C, Goyard J, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One 2014;9:e115060. [Crossref] [PubMed]
- Hamaker ME, Jonker JM, de Rooij SE, et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol 2012;13:e437-44. [Crossref] [PubMed]


