Family history of colorectal cancer and its impact on survival in patients with resected stage III colon cancer: results from NCCTG Trial N0147 (Alliance)
Introduction
Colon cancer is one of the most common forms of cancer worldwide and a leading cause of cancer-associated mortality. Amongst all cancers in both genders, colorectal cancer ranks third for incidence and death in the United States (1,2). In 2016 it is estimated that 134,490 people will be diagnosed with colorectal cancer and it will take the lives of 49,190 people (3).
Family history often portends increased risk of disease development. Familial clustering is commonly seen in colon cancer outside the context of defined genetic syndromes such as hereditary non-polyposis colorectal cancer (HNPCC) or familial adenomatous polyposis (FAP) (4,5). In colon cancer, a family history of disease results in a 2-fold increase in an individual’s risk of disease development (6-9). One meta-analysis of 59 studies calculated a pooled relative risk of 2.24 for individuals with one affected relative, rising to 3.97 with two or more affected relatives. These data equate to a population risk in a 50-year-old of 3.4% when an individual has one relative with disease compared to 1.8% when an individual has no family history of colon cancer (10). Risks were observed to be even greater for people with relatives who were young at the age of diagnosis and those with more than 2 relatives affected by disease (11,12). When an individual has a first-degree relative diagnosed at <50 years of age, multiple studies have reported a 3-fold increase in risk (13-15).
The influence of a positive family history of colorectal cancer on colon cancer recurrence and survival among patients with diagnosed disease remains relatively uncertain. Earlier studies out of Japan and the US have shown that a family history of colon cancer can be associated with lower mortality (16,17). A Japanese study analyzed outcomes and found an improved prognosis in those with a positive family history (16), and in another study, patients with a family history had a hazard ratio (HR) of 0.72 for death or cancer recurrence compared to patients with no family history (17). However, other studies have shown opposing results with a family history of disease leading to worse survival. One study from Iran purported that family history of cancer led to poorer survival outcomes; however, most participants in this study suffered from other types of gastrointestinal (GI) cancer, namely esophageal cancer, and the sample included only 15 patients with colon cancer (18). Another study conducted with the Utah Population Database found that family history of colon cancer had a negligible impact on patterns of survival (19). Thus, conflicting information exists on the association between a family history of colorectal cancer and survival outcomes. This study attempts to clarify the relationship between family history of colorectal cancer and survival outcomes through the use of a large and more homogeneous cohort of patients.
Methods
Study population
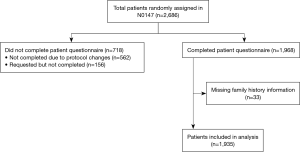
The patient cohort used in this study was derived from subjects enrolled in the North Central Cancer Treatment Group (NCCTG) clinical trial (N0147) entitled “A Randomized Phase III Trial of Oxaliplatin Plus 5-Fluorouracil/Leucovorin with or without Cetuximab after Curative Resection for Patients with Stage III Colon Cancer.” The NCCTG is now part of the Alliance for Clinical Trials in Oncology. This institutional review board approved, multicenter clinical trial—outlined in detail elsewhere—randomized patients with resected stage III colon cancer to standard adjuvant therapy (FOLFOX) plus or minus cetuximab (20). Patients were enrolled at multiple institutions across North America between February 10, 2004 and November 25, 2009. Enrollment occurred after written informed consent was obtained. Following enrollment patients completed family history and life-style questionnaires, which included information on family history of gastrointestinal (GI) cancers. Of the 2,686 subjects enrolled, 1,968 completed the patient survey, due to the questionnaire having been discontinued after the first 2,000 patients were enrolled. Of the subjects who completed the survey, 1,935 answered the questions about family history. Figure 1 provides an illustration of this study’s final sample size. KRAS, BRAF, and mismatch repair (MMR) status via molecular analysis were completed as a part of the study based on mandatory biospecimen collection.
Family history assessment
In the patient-completed questionnaires information on family history of cancer, comorbid conditions, certain exposures, life-style habits and physical characteristics was gathered (21). Information was requested on family size and selected medical history in blood-related relatives, including Barrett’s esophagus, Crohn’s disease, ulcerative colitis, colon polyps, esophageal cancer, stomach cancer, colon or rectal cancer, liver cancer, pancreatic cancer, breast cancer, uterine/cervical cancer, prostate cancer, and/or other cancers. Participants were asked to indicate if their relatives had been diagnosed with various medical conditions and to give the age they were first diagnosed. Options were given for “none of the above conditions” and “I don’t know this family member’s medical history”. Subjects were asked about their mother, father, maternal and paternal grandparents, siblings, and children. For each affected relative, age at diagnosis could be indicated in the following categories: 24 or younger, 25–49, 50–74, and 75+. The investigators did not attempt to validate the reported cancer cases in family members.
Tumor markers (KRAS, BRAF, MSI)
Tissue blocks from all study participants were obtained from the original surgical resection and assessed at Mayo Clinic for KRAS, BRAF, and MMR mutational status. DNA extracted from the tumor specimens was tested for KRAS status using DxS mutation kit, which checked for seven potential mutations in codons 12 and 13 (22). Testing for BRAF V600E was conducted with multiplex allele specific polymerase chain reaction-based assay and scored for the presence or absence of the V600E variant (23). Microsatellite instability (MSI) was determined through DNA MMR status. MMR status was assessed using immunohistochemical studies of hMLH-1, hMSH-2, and hMSH-6 proteins (24). Tumors were classified with defective MMR (dMMR) if they exhibited loss of expression of any of those three proteins. Tumors were classified with proficient MMR (pMMR) if they had all three proteins intact without loss of expression. These assays for KRAS, BRAF, and MMR tumor characterization were conducted without information on the patient, treatment, or outcome status.
Study end points
In this study, the primary clinical endpoint was disease-free survival (DFS), defined as the time from randomization to the first of either tumor recurrence or death from any cause. Secondary endpoints included time to recurrence (TTR) and overall survival (OS). TTR was defined as time from randomization to tumor recurrence. DFS and TTR were censored at 5 years. If participants died before a diagnosis of recurrence, for the TTR endpoint, they were censored at the time of their last disease evaluation date as documented by the treatment provider. OS was defined as time from randomization to death from any cause and censored at 8 years.
Statistical methods
Descriptive statistics were tabulated by family history status and compared between groups by Kruskal-Wallis and chi-square tests for continuous and categorical factors, respectively (25,26). The distributions of survival outcomes were estimated by the Kaplan-Meier method (27) and compared between groups by the log-rank test (28,29). Cox regression models were used to evaluate the adjusted associations between family history and time-to-event outcomes, adjusting for relevant baseline factors [age, sex, race, performance status, T/N stage, treatment arm, body mass index (BMI), histologic grade, tumor location, KRAS/BRAF mutation status, and MMR status] (30). The potential differential relationship between family history and outcomes per KRAS/BRAF mutation status and MMR status was tested by including interaction terms in the Cox models.
Since there was no significant interaction effect between family history and treatment assignment on outcomes (all interaction P values >0.31), the analysis was conducted by pooling the two treatments. Analyses were based on follow-up through August 5, 2015, and were performed by using SAS version 9.4 (SAS Institute, Cary, NC, USA). Two-sided P values less than 0.05 were considered statistically significant for main effects, while two-sided P values less than 0.01 were considered significant for interactions. All data collection and statistical analyses were performed by the Alliance Statistics and Data Center.
Results
The primary results from N0147 were previously published and no significant difference was observed in DFS between treatment arms (20).
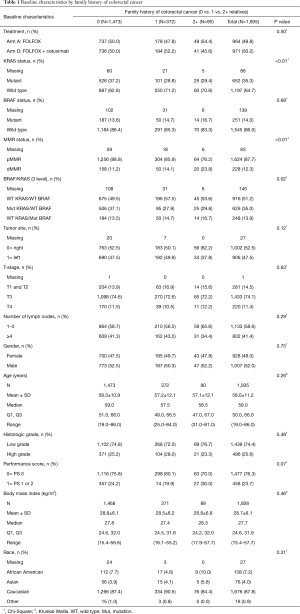
Baseline characteristics for study patients for whom family history data was obtained are presented in Table 1.
Full table
Of the 1,935 patients who responded to the questionnaire, 462 (23.9%) identified themselves as having a family history of colorectal cancer in at least one relative. Compared to participants without a family history, those with a positive family history were more likely to have tumors that were KRAS wild type (P≤0.01) and MMR deficient (P≤0.01), and have the combination of KRAS and BRAF wild type statuses (P=0.02). Other patient and tumor characteristics were similarly distributed among patients with and without a positive family history (see Table 1).
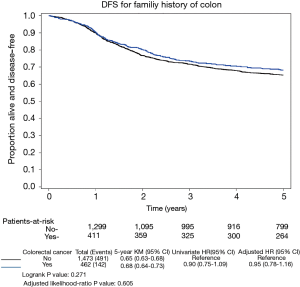
Median follow-up time was 6.8 years for those alive at last follow-up. Of the 1,935 patients, 561 experienced a recurrence of their cancer, 530 died from any cause, and 633 patients either had a recurrence or died. There was no difference in DFS outcomes in those with or without a family history of colorectal cancer as seen in Figure 2.
Family history of colorectal cancer was not associated with a significant difference in DFS, TTR, or OS as shown in Figure 3A, B, and C respectively.
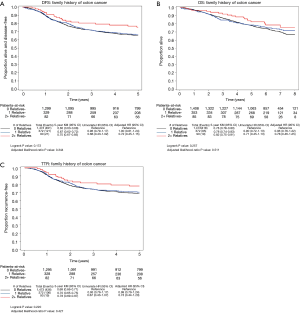
This relationship was also not statistically significant after adjusting for other variables (treatment arm, ECOG PS, T-stage, lymph nodes, sex, histologic grade, race, BRAF/KRAS, tumor site (proximal vs. distal), MMR status, age, and BMI). Patients with a positive family history had a multivariate HR of 0.95 [95% confidence interval (CI), 0.78–1.16; P=0.61] for DFS. Recurrence of cancer or mortality regardless of cause occurred in 142 of the 462 patients (31%) with a positive family history of colorectal cancer compared with 491 of the 1,473 patients (33%) with a negative family history.
The endpoints of TTR and OS were similar. Patients with a positive family history had a multivariate HR of 0.94 (95% CI, 0.76–1.16; P=0.57) for TTR. Cancer recurred in 125 of the 462 patients (27%) with a positive family history of colorectal cancer compared with 436 of the 1,473 patients (30%) with a negative family history. Patients with a positive family history had a multivariate adjusted HR of 0.92 (95% CI, 0.74–1.15; P=0.48) for OS.
The trend towards a slightly improved prognosis associated with family history was present with an increased number of relatives reporting a family history of colorectal cancer as shown in Figure 3A. Of the 462 patients with a positive family history, 372 reported one relative with colorectal cancer and 90 reported two or more affected relatives. Patients with a positive family history in one affected relative (vs. 0 relatives) had a multivariate adjusted HR of 1.00 (95% CI, 0.81–1.24) for DFS. In patients with a positive family history in two or more affected relatives (vs. 0) there was some reduction in risk with a multivariate adjusted HR of 0.72 (95% CI, 0.45–1.15). The log-rank P value was 0.17 (adjusted P value =0.344). Similar trends were noted in TTR and OS as demonstrated in Figure 3B and C respectively.
Similar results were also found when limiting the family history to first-degree relatives only (n=327). Specifically, no significant differences were observed for family history of colorectal cancer (yes vs. no) for DFS [adjusted HR 0.87; (95% CI, 0.69–1.10); adjusted P=0.23], TTR [adjusted HR 0.89; (95% CI, 0.69–1.14); P=0.35], or OS [adjusted HR 0.84; (95% CI, 0.65–1.09); P=0.19] (data not shown).
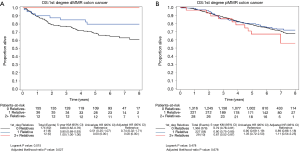
The interactions of KRAS, BRAF, and MMR status with family history for the time to event endpoints were also explored. The only significant interaction was for MMR and first-degree relatives with a family history of colorectal cancer (0 vs. 1 vs. 2+ relatives) for OS (univariate P=0.001), which remained significant even after adjusting for other factors (P=0.029). Family history was found to have a significant impact for patients whose tumors were MMR deficient (dMMR; adjusted P=0.027), while family history did not play a role for patients with MMR proficient tumors (pMMR; adjusted P=0.68) (Figure 4A and B).
For patients with dMMR tumors (Figure 4A), the 5-year survival was 100% for patients with a first-degree family history of 2 or more affected relatives (n=12) compared to 80% (95% CI, 68–93%) for patients with 1 affected relative (n=41) and 69% (95% CI, 62–76%) for patients with 0 affected relatives (n=174).
The association between DFS and family history across subgroups based on variables likely to impact patient outcome were explored and presented in Figure 5.
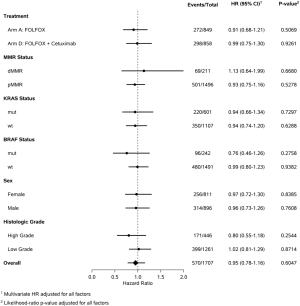
The lack of prognostic effect of family history on DFS was consistent across treatment arm, MMR, BRAF, KRAS, sex or histologic grade.
Discussion
This study explored the impact of a family history of colorectal cancer on measures of outcome in patients with resected stage III colon cancer. In this cohort of clinical trial participants with resected stage III colon cancer, a family history of colorectal cancer did not have a statistically significant impact on cancer recurrence and mortality. At most, a non-significant trend toward improved outcome was observed for patients with multiple (2+) family members who had a history of colorectal cancer, where observed outcomes for DFS, TTR, and OS were improved.
A family history of colorectal cancer is a well-documented risk factor for the development of colorectal cancer. However, previously published studies have shown conflicting findings in terms of the impact of family history on subsequent outcomes and survival amongst patients with resected colon cancer. A study from the Japanese registry (N=15,369) and a study by Chan and Meyerhardt (N=1,087) suggest a significant reduction in mortality and cancer recurrence when a family history of disease is present (16,17). These findings are in contrast to those by Slattery and Kerber (N=2,236), who reported that family history has no overall effect on survival (19). In our large study (N=1,935) with uniform standard of care treatment and meticulous follow-up, no statistically or clinically significant relationships between family history and outcomes were observed, raising doubt on the prior positive reports.
Our study found that first-degree relative family history has a significant impact on OS for patients whose tumors were MMR deficient (dMMR; adjusted P=0.027), while first-degree relative family history did not play a role for patients with MMR proficient tumors (pMMR; adjusted P=0.68) (univariate interaction P=0.001; adjusted interaction P=0.029). This prognostic relationship was known from earlier research and confirmed here as evidenced by Figure 4A and B (31). Colorectal tumors exhibiting MSI have a tendency to arise in the right side of the colon and manifest distinctive histologic features (32). They carry a better prognosis than microsatellite stable tumors and can respond differently to chemotherapy (33). Defective MMR and MSI are seen at a rate of 90% in HNPCC cases and approximately 10–15% in sporadic colorectal cancer cases (34-36). These results suggest that some participants included in our study may belong to families with HNPCC.
Data were also explored for a relationship between family history of non-colon GI cancers including esophageal, gastric, pancreatic, and liver cancers and survival outcomes. No significant relationship was found (data not shown). Outcomes including DFS, TTR, and OS appeared to be unaffected by a broader family history of GI cancer.
The strengths of our study include its large scale (N=1,935) as it stems from a North America, multicenter, phase III clinical trial. Additionally, as all patients had stage III disease as a criterion for enrollment, that qualifier thereby limited heterogeneity within the study cohort. By the design of the trial, treatment and follow-up were standardized, and treatment consisted of the present standard of care regimen of FOLFOX. Detailed information on family history was collected at the beginning of the study and the survival outcomes were recorded prospectively. All patients provided biospecimens that allowed for a complete analysis of the potential modifying impact of MMR, BRAF, and KRAS.
Limitations of the study include patient-reported family history status, which is subject to recall bias and errors in classification of history. This study’s questionnaire collected information on parents, grandparents, siblings and children. Increasing the degree of relatives has the potential to decrease the accuracy of the patient reported family health history. No attempt was made to validate the diagnosis in relatives. The study population was largely non-Hispanic whites and thus the results may not be generalizable to other racial or ethnic populations. It is likely, given the size of the study, that the sample population included some subjects with HNPCC or FAP. Although rare, these conditions do comprise about 5% of cases of colorectal cancer, which means that about 100 patients in our study could be from families affected by a genetic syndrome. These families may have their cancer diagnosed earlier since knowledge of increased risk can motivate patients to be screened, which in turn may impact subsequent survival (13).
The relationship between family history and colorectal cancer survival outcomes remains enigmatic and this study provides important findings to help clarify this conundrum. These findings suggest that if a trend in improved survival exists in patients with a family history of colorectal cancer it is manifest only in those patients with a family history of two or more relatives with colorectal cancer. These improved outcomes may correlate with defective MMR status, which we observed to interact significantly with family history to affect OS. In this subgroup of patients, family history of colorectal cancer in a first-degree relative was found to have a significant impact for patients with dMMR tumors, while family history did not play a role for patients with pMMR tumors. These findings would suggest dMMR as a possible pathway through which there is a connection, and further study is warranted.
Acknowledgements
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA025224, U10CA077658, U10CA180790, U10CA180850, and UG1CA189863.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Rim SH, Seeff L, Ahmed F, et al. Colorectal cancer incidence in the United States, 1999-2004: an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115:1967-76. [Crossref] [PubMed]
- Potter JD, Slattery ML, Bostick RM, et al. Colon cancer: a review of the epidemiology. Epidemiol Rev 1993;15:499-545. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919-32. [Crossref] [PubMed]
- Houlston RS, Peto J. Genetics of common cancers. In: Eeles RA, Ponder B, Easton DE, et al. editors. Inherited predisposition to cancer. Chapter 13. London: Chapman and Hall, 1996:208-26.
- Fuchs CS, Giovannucci EL, Colditz GA, et al. A prospective study of family history and the risk of colorectal cancer. N Engl J Med 1994;331:1669-74. [Crossref] [PubMed]
- St John DJ, McDermott FT, Hopper JL, et al. Cancer risk in relatives of patients with common colorectal cancer. Ann Intern Med 1993;118:785-90. [Crossref] [PubMed]
- Hemminki K, Li X. Familial colorectal adenocarcinoma from the Swedish Family-Center Database. Int J Cancer 2001;94:743-8. [Crossref] [PubMed]
- Goldgar DE, Easton DF, Cannon-Albright LA, et al. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst 1994;86:1600-8. [Crossref] [PubMed]
- Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer 2006;42:216-27. [Crossref] [PubMed]
- Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol 2001;96:2992-3003. [Crossref] [PubMed]
- Lovett E. Family studies in cancer of the colon and rectum. Br J Surg 1976;63:13-8. [Crossref] [PubMed]
- Taylor DP, Burt RW, Williams MS, et al. Population-based family history-specific risks for colorectal cancer: a constellation approach. Gastroenterology 2010;138:877-85. [Crossref] [PubMed]
- Slattery ML, Kerber RA. Family history of cancer and colon cancer risk: the Utah Population Database. J Natl Cancer Inst 1994;86:1618-26. [Crossref] [PubMed]
- Negri E, Braga C, La Vecchia C, et al. Family history of cancer and risk of colorectal cancer in Italy. Br J Cancer 1998;77:174-9. [Crossref] [PubMed]
- Clinical and pathological analyses of patients with a family history of colorectal cancer. Registry Committee, Japanese Research Society for Cancer of the Colon and Rectum. Jpn J Clin Oncol 1993;23:342-9. [PubMed]
- Chan JA, Meyerhardt JA, Niedzwiecki D, et al. Association of family history with cancer recurrence and survival among patients with stage III colon cancer. JAMA 2008;299:2515-23. [Crossref] [PubMed]
- Ghadimi M, Mahmoodi M, Mohammad K, et al. Family history of the cancer on the survival of the patients with gastrointestinal cancer in northern Iran, using frailty models. BMC Gastroenterol 2011;11:104. [Crossref] [PubMed]
- Slattery ML, Kerber RA. The impact of family history of colon cancer on survival after diagnosis with colon cancer. Int J Epidemiol 1995;24:888-96. [Crossref] [PubMed]
- Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383-93. [Crossref] [PubMed]
- Patient Questionnaire. A Randomized Phase III Trial of Oxaliplatin (OXAL) Plus 5-Fluorouracil (5-FU)/Leucovorin (CF) with or without Cetuximab (C225) after Curative Resection for Patients with Stage III Colon Cancer. NCCTG. Appendix IX. Study N0147.
- O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549-56. [Crossref] [PubMed]
- Domingo E, Laiho P, Ollikainen M, et al. BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet 2004;41:664-8. [Crossref] [PubMed]
- Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 2002;20:1043-8. [Crossref] [PubMed]
- Kruskal WH, Allen Wallis WA. Use of Ranks in One-Criterion Variance Analysis. J Am Stat Assoc 1952;47:583-621. [Crossref]
- Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed. New York, NY: John Wiley & Sons, 1981.
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958;53:457-81. [Crossref]
- Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J Royal Stat Soc Series A 1972;135:185-207. [Crossref]
- Therneau T, Grambsch P. Modeling Survivor Data. New York, NY: Springer, 2000.
- Cox DR. Regression models and life-tables. J Royal Stat Soc Series B (Methodological) 1972;34:187-220.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609-18. [Crossref] [PubMed]
- Sinicrope FA, Rego RL, Foster N, et al. Microsatellite instability accounts for tumor site-related differences in clinicopathologic variables and prognosis in human colon cancers. Am J Gastroenterol 2006;101:2818-25. [Crossref] [PubMed]
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26. [Crossref] [PubMed]
- Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993;260:812-6. [Crossref] [PubMed]
- Aaltonen LA, Peltomäki P, Mecklin JP, et al. Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res 1994;54:1645-8. [PubMed]
- Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816-9. [Crossref] [PubMed]