Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal and hepatic metastases: a case-control study
Introduction
A slow shift in treatment is underway in the area of colorectal peritoneal metastases (PM). Cytoreductive surgery (CRS) and intraperitoneal chemotherapy (IPC) are becoming a valid treatment option for this loco-regional disease; particularly the hyperthermic type of IPC known as hyperthermic intraperitoneal chemotherapy (HIPEC). A recent study on CRS and IPC published a median overall survival of 34 months in patients with non-gynecological malignancies with PM (1). There are a few reports with survival results of this procedure being combined with hepatic resections for hepatic metastases (HM) in an attempt to reach a disease free patient (2-6). Furthermore, there are two large multi-centre studies that have included hepatic metastases in their multivariable analyses (without any specific clinical or survival data). Both studies come from France and one shows that concomitant HM is a definite negative prognostic factor for overall survival while the other one shows no statistical difference in survival (7,8).
One review article from 2009 concluded that while there may be some evidence of a survival benefit, the evidence at hand is too scarce to make any general recommendations (9). Further studies are needed to elucidate the value of treating colorectal PM and HM aggressively with surgery. The aim of this study was to compare the treatment of colorectal PM with CRS and IPC vs. the treatment of colorectal PM and HM with CRS, IPC, and hepatic resections. The overall survival, disease free survival, morbidity, and mortality were the parameters of main interest.
Patients and methods
Patient selection
From the Uppsala University Hospital prospective database of colorectal PM, all patients undergoing simultaneous PM and HM treatments were extracted and included in the study’s PM/HM group. A second control group (PM only) was selected without knowledge of survival by matching 1:2 for the following parameters: HIPEC or sequential postoperative intraperitoneal chemotherapy (SPIC), R1 or R2 resections, and peritoneal cancer index (PCI) (same PCI ±1 point). If more than 2 patients were eligible for matching than the two patients with the closest treatment date to the PM/HM patient were chosen. Clinicopathological variables were collected retrospectively from the patient charts as well as surgical variables from the operation notes. The 90-day morbidity and in-hospital treatment-related morbidity was reported according to Common Terminology Criteria for Adverse Events v3.0 and only grades III to V adverse events were registered. The study was approved by the Uppsala Regional Ethics board.
Surgical methods
The CRS was performed as previously described with different organ resections where needed combined with peritonectomy procedures of affected peritoneum (10). The aim was to reach macroscopic complete resection of the disease which was designated as an R1 resection. Where there was macroscopic disease remaining, the patients was designated an R2 resection. The PCI is a semi-quantitative score that combines tumour nodule size with distribution according to 13 abdominal regions and is determined during the opening phase of surgery. Each region can have a score from zero to three, depending on nodule size; thus, the top score with maximal tumour size and distribution is 39 (11). The prior surgical score (PSS) is a measure of the extent of surgical trauma prior to the CRS and IPC treatment (11).
In the PM/HM group, the HM were determined preoperatively by a computer tomography scan. The surgical techniques used were classified as either segmental resections (involving 1 or more segments), or hemihepatectomies (where either the whole left or right lob was resected).
Intraperitoneal chemotherapy methods
Two methods of IPC have been in use at the Uppsala Centre, HIPEC and SPIC. HIPEC was performed according to the colosseum method as previously described (12). Briefly, a Tenckhoff inflow catheter was centrally placed in the abdomen and four outflow catheters were inserted through separate stab incisions through the abdominal wall. Both the inflow and outflow catheters were connected to a perfusion pump and a heat exchanger. The skin of the abdomen was attached to a retractor ring and covered with a plastic film. The intra-abdominal temperature measured with three thermal probes was maintained at 41-42 °C with a flow rate of 1-2 L/min. Electrolyte-free glucose (50 mg/mL) was used for the oxaliplatin perfusion. Before perfusion, the body temperature was lowered to 35 °C with a cooling blanket (Allon 2001 Thermowrap, MTRE Advanced Technology Ltd. Yavne, Israel). The HIPEC treatment consisted of oxaliplatin 460 mg/m2 for 30 min intraperitoneally combined with 5-fluorouracil (5-FU) 400 mg/m2 and Leucovorin 60 mg/m2 intravenously (n=13) or oxaliplatin 360 mg/m2 and irinotecan 360 mg/m2 for 30 min intraperitoneally combined with 5-FU 400 mg/m2 and Leucovorin 60 mg/m2 intravenously (n=7). In eight patients the HIPEC was combined with early postoperative chemotherapy (EPIC) administered through the drains placed at the end of the surgery. The EPIC treatment consisted of 5-FU 550 mg/m2 intraperitoneally and Leucovorin 60 mg/m2 intravenously. In Table 1, the IPC treatment is detailed.
The SPIC patients received a PORT A CATH (No. 21-2000-04, SIMS Deltec, Inc., St Paul, MN, USA) placed subcutaneously above the periost of the lower ribs with the catheter tunnelled through the abdominal wall and directed towards the principal tumour site (13). A SPIC treatment cycle consisted of 5-FU 500-600 mg/m2 administered intraperitoneally and leucovorin 60 mg/m2 administered intravenously once a day for six days. A total of eight cycles of SPIC were planned with 4-6 week intervals between the cycles as an adjuvant treatment over a 6-month period.
Statistics
Comparisons of categorical variables between patients with PM/HM and PM alone were evaluated with Pearson’s χ2 test and continuous variables with the Mann-Whitney U test. Survival data were represented by a Kaplan-Meier curve and differences were calculated with the two-tailed log rank test. Median survival was taken from the curve and the confidence interval of the median was calculated. Patients were considered censored if they died of causes other than cancer or if they were still alive at the last check up. Statistical significance was set at P<0.05.
A univariate Cox regression analysis for overall survival was used to assess the prognostic value of variables through hazard ratios. A multivariable Cox regression model was constructed using variables from the univariate analysis with a P≤0.1. There were 3 variables chosen to be included in the multivariable analysis. The stability of the analysis is borderline as it is generally accepted that for each variable in the analysis there should be 10 completed cases. This study had 18 death events and as such the analysis had 6 death events per variable (14). Calculations were made with STATISTICA 10 software.
Results
Participants
From the CRS and IPC database between 1994 and 2010, there were 11 patients with concomitant treatment of colorectal PM and HM in one procedure (CRS + hepatic resection). All of these patients were included in a PM/HM group. There were 140 remaining patients in the database after extracting the PM/HM group. A selection process was conducted according to the methods section. Successful 1:2 matching was able to be performed according to HIPEC/SPIC, R1/R2 resection, and PCI (maximum point difference of 1) which amounted to 22 patients for the PM only group. Thus, the total study size was 33 patients.
Clinical and surgical results
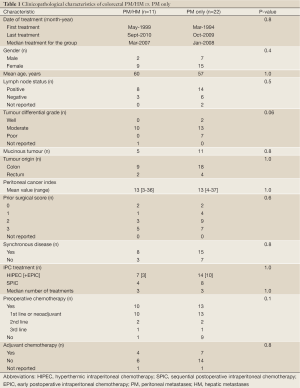
Baseline clinical and surgical characteristics are presented in Tables 1,2. There was only one statistical difference which was the number of gastrointestinal resections. One other variable came close with a P-value of 0.06 concerning the greater number of low differential tumours in the PM only group. The median number of SPIC treatments received was three in each group. All HIPEC treatments were completed as planned. The median number of HM lesions was 1. Three patients were treated for 2 lesions and one patient was treated for 3 lesions.
Full Table

Full Table
Preoperative chemotherapy was not significantly different but had a numerical tendency (Table 1) while the adjuvant treatment did not differ at all. In the PM/HM group, there were 17 lines of systemic chemotherapy administered from diagnosis till CRS and IPC treatment including adjuvant treatment. There were 9 oxaliplatin based treatments, 6 irinotecan, and 2 5-FU. A combination with biological therapy was given in 4 lines - 3 with bevacizumab and 1 with cetuximab. In the PM group, there were 23 lines of systemic chemotherapy during the same interval from diagnosis to adjuvant treatment. There were 14 oxaliplatin based treatments, 3 irinotecan, and 3 5-FU. There was missing data as to the drugs administered in 3 adjuvant treatments. A combination with biological therapy was given in 5 lines—3 with bevacizumab and 2 with cetuximab.
Recurrence results after R1 resection
In the PM/HM group, there were 10 patients with R1 resections and 8 of these patients recurred: 1 patient recurred with isolated PM, 3 patients recurred with isolated HM, 3 patients recurred with both PM and HM, and there was insufficient data concerning the location of a recurrence in 1 patient. There was 1 postoperative death and 1 patient with no recurrences at 42 months.
In the PM group, there were 20 patients with R1 resections and 11 of these patients recurred: 2 patients recurred with isolated PM, 3 patients developed isolated HM, 1 patient developed isolated pulmonary metastases, 2 patients developed both PM and HM, 2 patients developed both HM and pulmonary metastases, and there was insufficient data concerning the location of a recurrence in 1 patient. There were 9 patients with no recurrences at 18, 20, 22, 27, 31, 32, 32, 68, and 138 months.
Concerning peritoneal recurrences, there were 36% recurrences in the PM/HM group and 18% in the PM group (P=0.3). Concerning hepatic recurrences, there were 55% recurrences in the PM/HM group and 32% in the PM group (P=0.2). Currently, only 1/10 patients with R1 resection remain disease-free in the PM/HM group, while 9/20 patients with R1 resection remain disease free in the PM group (P=0.05).
Overall survival and prognostic factors
The median follow-up time was 57 months for the PM/HM group and 45 months for the PM group. The PM/HM group had a median overall survival (OS) of 15 months (95% CI: 6-46 months) and the PM group had a median OS of 34 months (95% CI: 19-37 months) as seen in Figure 1 (P=0.2). The disease free survival (DFS) was 10 months (95% CI: 3-14 months) for the PM/HM group and 24 months (95% CI: 10-32 months) for the PM group (P=0.1). The three-year OS was 30% in the PM/HM group and 47% in the PM group and the three-year DFS was 20% and 42%, respectively.
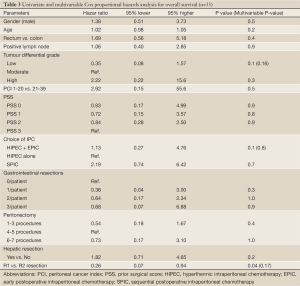
There was only 1 univariate prognostic factor which was significant and it was the R1 resection variable. It did not maintain an independent prognostic value which is probably due to the fact that only 3 patients in the entire study were R2 resections and the rest R1. HM was not a negative prognostic factor in the prognosis analysis (Table 3).
Full Table
Morbidity and mortality
There were 3 patients (27%) with grade III-IV morbidity in the PM/HM group and 6 patients (27%) in the PM group (P=1.0). There was one postoperative mortality in the PM/HM group and none in the PM group. The most common morbidity was postoperative infections requiring intravenous antibiotics. Only one fistula occurred in the study (PM only group).
Discussion
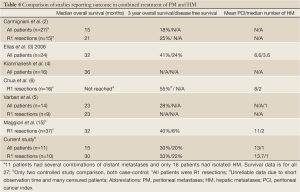
This is a matched comparison of colorectal PM/HM treatment vs. PM treatment alone. Considering the tendency towards worse DFS and a significantly increased recurrence rate, the concomitant presence of HM should definitely be considered a negative prognostic factor even when only a solitary HM is present. This conclusion is corroborated by the only other matched study performed in this area which was recently published from France (Table 4). This study is the largest one to date with only 37 patients (matched to 61 PM patients). The presence of concomitant HM was shown to be an independent prognostic factor. The three-year disease-free survival was poor at only 6%. Their conclusion was that synchronous PM and HM disease was feasible to operate but that the PCI score should be lower than 12 and that the number of HM should be max 2. This differs from the earlier Milano consensus, which puts the limit at three (16).
Full Table
The aim of our study was to provide matched groups according to the most important prognostic indicators: PCI, surgical result, and type of IPC (7,8,17). The matching was successful and comparison of clinical and surgical variables was highly congruous. Besides the difference of HM, only one out of the other 26 variables was statistically different (number of gastrointestinal resections) and only one other variable was close at P=0.06 (more low tumour grade in the PM group). In order to ascertain the effect of these differences a univariate and a multivariable Cox proportional hazard regression was performed. Both the number of gastrointestinal resections and tumour grade had no statistically significant effect on the overall survival (Table 3).
After these analyses results, the effect of HM on the overall survival was evaluated. Two methods were used, the two-tailed log rank test of a Kaplan-Meier curve (Figures 1,2) and the Cox proportional hazard regression (Table 3). The overall survival did not appear to be statistically effected by the presence and concomitant treatment of HM; but, the study does not have enough power to ascertain this adequately. On the other hand, there was a clear tendency toward lower DFS in the PM/HM group as seen in Figure 2. When comparing recurrences between the groups, it becomes increasingly clear that there is a significantly higher risk of recurrence in the PM/HM group. Currently, only 1/10 (10%) R1 resections in the PM/HM group remains disease free, while 9/20 (45%) is disease free in the PM group (P=0.05). Furthermore, it is interesting that the PM/HM group recurs almost twice as much regardless of location compared to the PM group. This is an interesting finding as it supports the notion that some patients with isolated PM disease may have a different metastatic profile and potential. One may speculate that the genetic mutations needed for hematogenic growth has not yet been acquired in many patients with isolated PM. This may also be the reason that some patients become eligible for repeat cytoreductive procedures (18).
Most studies report on the overall survival as seen in Table 4. Now, this is, of course, a relevant aspect. However, even systemic chemotherapy is now producing low 5-year survival rates, but still with very few long term disease free survivals (19,20). This is the main difference between these two alternatives. The aggressive cytoreductive approach is intended to cure. If the curative potential is lost, the validity of the treatment at least for colorectal cancer could be questioned. The massing evidence for CRS and IPC treatment of isolated PM disease is now showing an excellent 5-year disease-free survival between 15% and 32% (18). This needs to be the main aim when reviewing results from colorectal PM/HM as well. There needs to be a curative potential for this treatment to remain as a valid treatment option. Unfortunately, few studies report the disease-free survival. There is a need for change here. As more centres are now starting to apply the concepts of CRS in colorectal PM disease, there will come more reports on colorectal PM/HM as well. As such, it is important in future studies to keep the disease free survival a key aspect of the outcome reporting.
It is also noteworthy that our study hade a significantly higher mean PCI score than the others studies (Table 4). However, the R1 resections still produced similar results as the other studies despite the increase in mean PCI. Since the consensus statement from Milano stated that concomitant HM with 1-3 metastases appears to not affect the overall survival of colorectal PM, our institution has implemented this in clinical practice (16). We have previously not performed laparoscopic staging and high PCI values have not automatically been cause for exclusion or open-and-close. Instead, at exploration a decision is made by the surgeon whether or not it is technically possible to reach a CC 0 score regardless of the PCI. Other institutions have had other policies (3,6); and for this reason, this study has a significantly higher mean PCI score. Despite this, results from our institution remain optimistic, but further investigations are needed particularly to determine if long term disease-free survival is achievable. This is a necessity if the treatment is to be successful in combined colorectal peritoneal and hepatic metastases. Since our study showed that concomitant HM appears to affect recurrence rates and disease free survival, one cannot assume that the same improvement over systemic chemotherapy exists as it does for PM alone. Therefore, there is a need to re-evaluate this treatment option for combined PM/HM disease. A randomised trial between systemic chemotherapy vs. CRS, IPC, and hepatic resections is called for. Furthermore, the Milano consensus may need to be revised as new evidence is brought forth demonstrating the negative prognostic impact of concomitant hepatic disease.
In conclusion, concomitant treatment of PM and HM with CRS/IPC/hepatic resections is feasible with no increase in morbidity or mortality, but the risk of recurrences is significantly higher in the PM/HM group with a tendency towards worse DFS. The curative potential of the combination treatment of PM/HM needs to be elucidated in future studies, perhaps where possible in phase III randomized trials (systemic chemotherapy vs. CRS, IPC and hepatic resections).
Acknowledgements
ALF funding from the Uppsala University Hospital was used in the production of this article.
Disclosure: The authors declare no conflict of interest.
References
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 2010;116:5608-18. [PubMed]
- Carmignani CP, Ortega-Perez G, Sugarbaker PH. The management of synchronous peritoneal carcinomatosis and hematogenous metastasis from colorectal cancer. Eur J Surg Oncol 2004;30:391-8. [PubMed]
- Elias D, Benizri E, Pocard M, et al. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur J Surg Oncol 2006;32:632-6. [PubMed]
- Kianmanesh R, Scaringi S, Sabate JM, et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg 2007;245:597-603. [PubMed]
- Varban O, Levine EA, Stewart JH, et al. Outcomes associated with cytoreductive surgery and intraperitoneal hyperthermic chemotherapy in colorectal cancer patients with peritoneal surface disease and hepatic metastases. Cancer 2009;115:3427-36. [PubMed]
- Chua TC, Yan TD, Zhao J, et al. Peritoneal carcinomatosis and liver metastases from colorectal cancer treated with cytoreductive surgery perioperative intraperitoneal chemotherapy and liver resection. Eur J Surg Oncol 2009;35:1299-305. [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [PubMed]
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63-8. [PubMed]
- Carpizo DR, D’Angelica M. Liver resection for metastatic colorectal cancer in the presence of extrahepatic disease. Lancet Oncol 2009;10:801-9. [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [PubMed]
- Sugarbaker P. eds. Management of Peritoneal Surface Malignancy Using Intraperitoneal Chemotherapy and Cytoreductive Surgery. A Manual for Physicians and Nurses. Grand Rapids, MI: The Ludann Company, 1998.
- Mahteme H, Hansson J, Berglund A, et al. Improved survival in patients with peritoneal metastases from colorectal cancer: a preliminary study. Br J Cancer 2004;90:403-7. [PubMed]
- Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503-10. [PubMed]
- Maggiori L, Goéré D, Viana B, et al. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent?: a case-control study. Ann Surg 2013;258:116-21. [PubMed]
- Esquivel J, Elias D, Baratti D, et al. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol 2008;98:263-7. [PubMed]
- Cashin PH, Graf W, Nygren P, et al. Intraoperative hyperthermic versus postoperative normothermic intraperitoneal chemotherapy for colonic peritoneal carcinomatosis: a case-control study. Ann Oncol 2012;23:647-52. [PubMed]
- Cashin PH, Graf W, Nygren P, et al. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: prognosis and treatment of recurrences in a cohort study. Eur J Surg Oncol 2012;38:509-15. [PubMed]
- Dy GK, Krook JE, Green EM, et al. Impact of complete response to chemotherapy on overall survival in advanced colorectal cancer: results from Intergroup N9741. J Clin Oncol 2007;25:3469-74. [PubMed]
- Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 2012;30:263-7. [PubMed]


