Targeted therapy in biliary tract cancers—current limitations and potentials in the future
Introduction
Biliary tract cancers (BTC)/Cholangiocarcinoma (CCA) are resulting from malignant transformation of epithelial cells within the bile system as related but distinct malignancies along the intrahepatic and extrahepatic biliary tree. Besides the gallbladder carcinoma (GBC), CCA is also refers to cancers of the entire biliary tree. CCA is commonly classified as intrahepatic cholangiocarcinoma (iCCA) and extrahepatic cholangiocarcinoma (eCCA), with extrahepatic further divided into perihilar cholangiocarcinoma (pCCA) and distal cholangiocarcinoma (dCCA) tumors based on anatomical location with the cystic duct as the point of distinction (1). There is increasing recognition that these subtypes separated by anatomic origins of BTCs distinct not only in desmoplasia markers of cholangiocytes, but also in biological and clinical characteristics (2). BTC has an aggressive natural course without early specific warning sign of the disease; therefore, potential curative surgery is only suitable for a limited number of patients. So far, systemic treatment is confined to the gemcitabine and cisplatin combination as the practice standard for patients with advanced and metastatic disease (3). The overall outcome is disappointing with limited response rate (RR) and low 5-year survival rate. It is crucial to understand BTC/CCA carcinogenesis, tumor-stroma interactions, and key molecular pathways, and herald to establish targeted, individualized therapies for the heterogeneous disease, and eventually to improve the survival and overall outcomes of patients. In this review, we are going to summarize the results of systemic cytotoxic chemotherapy, and review the data from studies with ‘target-oriented’ agents. Since most of these studies are not targeting the distinguished subgroups of the disease specifically, they are more likely as ‘target intended’ rather than ‘target-oriented’. We will also discuss the current knowledge regarding the genetic basis of this disease, including molecular pathways involved in its carcinogenesis, and potential targeted therapies that hold promise in the future research and practice.
Cytotoxic chemotherapy
Cytotoxic chemotherapy remains the mainstay of treatment for patients with advanced unresectable or metastatic BTC. Given the rarity of this disease, clinical studies have been small and have almost always been combined with ‘lumping’ various BTCs with very few randomized trials have been conducted. The majority of trials have been performed with either fluoropyrimidine-based or gemcitabine-based combination. 5-flourouracil (5-FU) had been tested in small trials, both as monotherapy and in combinations. Overall RRs in these studies varied from 10% to 40%; median survival also varied notably, from 5 to 16 months (4-21).
A phase III study randomized 54 patients with previously untreated advanced biliary cancer between ECF (epirubicin, cisplatin, 5-FU) and FELV (5-FU/LV, etoposide) (14). The median OS was not significantly different between the two arm (9.02 months in ECF vs. 12.03 months in FELV, P=0.2059). Objective RRs were also similar (19.2% in ECF vs. 15% in FELV, P=0.72). The interesting point is greater than 60% of patients in each arm demonstrated resolution of pain, anorexia, weight loss, and nausea.
Based on the data of studies from advanced and metastatic pancreatic cancer, gemcitabine has also been evaluated biliary cancers at the similar setting (7,12,15,16,22-41) (Table 1). As a single agent, gemcitabine showed only moderate efficacy with RRs ranging from 0% to 30% at varied dosing schemes demonstrated. Efforts had been tried in combination of gemcitabine with multiple other cytotoxic agents, including 5-FU, capecitabine, cisplatin, oxaliplatin, and irinotecan with significant variations in RRs and survival.
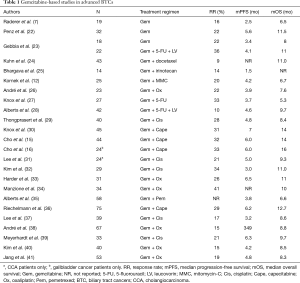
Full table
In order to assess the overall efficacy of systematic chemotherapy, a pooled analysis was performed including 104 trials published between 1985 and 2006 (42). The overall RR was 22.6%; and the tumor control rate (TCR) was 57.3%. Significant correlations of RR and TCR with survival times were found. Subgroup analysis showed superior RRs for GBC compared with CCA (RR 35.5% vs. 17.7%, P=0.008), but shorter OS for GBC (median 9.3 in CC vs. 7.2 months in GBC, P=0.048). Based on treatment type subgroup analyses, the analysis showed that regimens containing both gemcitabine and a platinum agent had significantly higher response and TCRs compared to either fluoropyrimidine or gemcitabine monotherapy or fluoropyrimidine-plus-platinum regimens. Based on this information, United Kingdom-based Advanced Biliary Care (ABC)-02 trials was conducted to validate the combination (3). In this study, 410 patients with non-resectable, recurrent, or metastatic BTC were randomized to receive either gemcitabine alone or gemcitabine and cisplatin combination. The trial included patients with CCA, gallbladder cancer, or ampullary cancer. Patients in the combination arm received cisplatin 25 mg/m2 and gemcitabine 1,000 mg/m2 on days 1 and 8 of a 3-week cycle, for eight cycles; patients in the gemcitabine monotherapy arm received gemcitabine 1,000 mg/m2 on days 1, 8, and 15 of a 4-week cycle, for six cycles. After a median follow-up of 8.2 months, the median OS was 11.7 months in the gemcitabine/cisplatin combination arm, compared to 8.1 months in the gemcitabine arm (HR =0.64, P<0.001). The median progression-free survival (mPFS) was improved with the combination (8.0 vs. 5.0 months; HR =0.64; P<0.001) as well. Severe hematologic toxicities were seen more frequently in the combination arm. However, there were more severe liver toxicities reported in the gemcitabine-alone arm for unclear reason.
Target intended therapy
Although the combination of gemcitabine and cisplatin has achieved some advances in the treatment of advanced and metastatic biliary track cancers and has been accepted as the standard treatment option as the first line therapy since 2010, the overall outcome is still disappointing. Clinic studies of target-oriented agents (most of them in combination with gemcitabine based regimen) have been attempted for improving the outcomes of the disease. Those target-oriented agents primarily are monoclonal antibodies and tyrosine kinase inhibitors against EGFR and vascular endothelial growth factor (VEGF) (43-57) (Table 2). However, no or only marginal benefits showed from those trials, which is likely because of a mixed cohort of BTC patients (iCCA, eCCA, and GBC), and the underlying genetic variability of the disease.
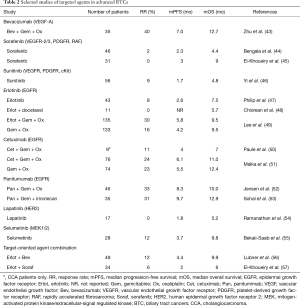
Full table
One phase III trial randomized 268 patients with metastatic biliary tract adenocarcinoma to the combination of gemcitabine and oxaliplatin (GemOx) with erlotinib or chemotherapy (GemOx) alone (49). The combination showed increased RR (30% vs. 16%, P=0.005), however, there were no significant difference in mPFS (5.8 vs. 4.2 months, P=0.087) and median overall survival (mOS) (9.5 months for both arms). A randomized phase II study evaluated the GemOx with or without cetuximab in 150 patients with advanced and metastatic BTC (51). There were no differences between the two arms in RR, mPFS or mOS. The TCOG trial evaluated the same regimen in advanced and metastatic BTC patients with stratification of iCCA/eCCA/GBC (71.3%/16.4%/12.3%) and KRAS status (36.1% KRAS mutation). On the intent-to-treat analysis, it showed some benefit of mPFS in the arm of GemOX + cetaximab (7.1 vs. 4.0 months, P=0.0069), but no difference in RR (27.3% vs. 15%, P=0.1223), and mOS (10.3 vs. 8.8 months, P=0.4057). Planned subgroup analysis showed that potential more benefits of cetaximab with GemOX in KRAS mutated patients with mPFS of 7.1 vs. 1.9 months (P=0.0351), however, no statistical significance in mOS (10.3 vs. 6.6 months, P=0.6924) (58). Interestingly, a pooled analysis with 161 trials comprising 6,337 patients (trials published in English between 1/2000 and 1/2014 as well as ASCO abstracts 2010 to 2013) showed some potential survival benefits of gemcitabine-based chemotherapy with targeted therapy (predominantly EGFR inhibitor as either monoclonal antibody or TKI) (59).
New insights into the molecular pathogenesis and therapeutic opportunities
Recent studies have begun to uncover the genetic and molecular processes underlying carcinogenesis using advanced technologies such as next-generation sequencing (NGS) in the BTC. The emerging knowledge and data generated from studies of epidemiology, genome profiling, and laboratory based investigations provide new insights into risk factors, genomic composition, cellular origins and contribution of the tumor microenvironment to the pathogenesis of BTC. The remarkable genetic heterogeneity of BTC may be the result of a complex interplay among different factors—some of them are shared by most human cancers, while others may be unique for this malignancy. Emerging data supports that iCCA, eCCA, and GBC represent distinct tumors arising from different genetic backgrounds (60,61).
Cancer-associated fibroblasts (CAF) in the tumor stroma
CAF secondary to desmoplastic response is a prominent tumor microenvironment characteristics of biliary track cancers, especially iCCA. CAF may impede access of therapeutic agents to the tumor and pose therapeutic challenges further besides the genetic heterogeneity of this malignancy (1,62). Preclinical studies have demonstrated a reduction in fibrosis and carcinogenesis in BTC/CCA with 1D11, a transforming growth factor β (TGF-β) antagonist, as well as curcumin, a nutraceutical agent (63). A pre-clinic study with an orthotopic CCA model showed that the BH3 (BCL-2 family protein, pro-apoptosis) mimetic, Navitoclax, enhanced selective CAF apoptosis, decreased expression of α-SMA, and reduced tumor burden and metastasis while improving survival (64). Further preclinical and clinical studies are needed to explore the role of antifibrotic therapies in CCA chemoprevention.
Inflammation and carcinogenesis
Chronic inflammation plays a significant role in the development of BTC. Chronic inflammatory pathways not only are key components in carcinogenesis, but also promote tumor invasion and migration. Primary sclerosing cholangitis (PSC), hepatobiliary flukes Opisthorchis viverrini (O. viverrini) and Clonorchis sinensis characterized by chronic biliary tract inflammation and liver injury, are common predisposing conditions for BTC. In addition, prolonged hepatolithiasis may promote CCA development by calculi occurring proximal to the hepatic duct confluence (2,65). Inducible nitric oxide synthase (iNOS) activation by inflammatory cytokines contributes to nitrosative stress by generation of excess nitric oxide, which then results in inhibition of DNA repair proteins, and single-stranded, double-stranded, and oxidative DNA lesions. Oxidative stress via generation of oxysterols, cholesterol oxidation products present in human bile, creates a milieu favorable for tumor development and progression by activating Hedgehog signaling pathway. Oxysterols, bile acids, and iNOS all stimulate over-expression of cyclooxygenase-2, which has been implicated carcinogenesis of BTC (66-68). Therefore, inflammatory processing control may play a significant role in management of BTC, especially in the prevention.
Genomic profiling studies
Genomic profiling has demonstrated characteristic profiles for iCCA and eCCA. Next generation sequencing (NGS) of a BTC series showed key variations in certain mutations based on tumor location (69). Mutations in the isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) genes have consistently been shown to be more frequent in iCCA versus eCCA or GBC. IDH mutations caused inhibition of HNF-4α (hepatocyte nuclear factor 4 alpha, a nuclear receptor; also known as NR2A1- nuclear receptor subfamily 2, group A, member 1), leading to impaired hepatocyte differentiation and increased cell proliferation, and associated with poorly differentiated histology. FGFR (Fibroblast growth factor receptor) mutations, specifically in FGFR2 are associated with the production of two fusion kinase genes, FGFR2-AHCYL1 and FGFR2-BICC1 that are mutually exclusive with KRAS/BRAF mutations. The median cancer specific survival is suggested significantly longer for patients whose tumors contained FGFR2 translocations (70).
It appears that mutational profiles may be influenced by the etiology of BTC. A study revealed the results of exome sequencing of 209 CCA samples from Asia and Europe with 108 cases of O. Viverrini infection related and the other 101 cases has non-O. viverrini-related etiologies (71). The study showed that TP53 was mutated in 40% of O. viverrini infection related CCA, and only in 9% of the non-O. viverrini-related cases (P<0.001). On the other hand, SMAD4 mutation was more frequently in non-O. viverrini-related CCA (6% vs. 19%, P=0.006). IDH1 and IDH2 mutations were tested in 22.2% of non-O. viverrini iCCA and only 3.2% of O. viverrini-related iCCA.
Activating mutations in cell proliferation oncogenes lead to uncontrolled cell growth and survival. The Ras/MAPK signaling pathway plays a key role in cell growth, differentiation, survival, and migration. Gain-of-function mutations in KRAS are present in approximately 45–55% of iCCA and 10–15% of eCCA. One study showed that BRAF, an important downstream effector of KRAS, was found to be mutated in 22% of iCCA, no BRAF mutation was found in those cases with KRAS mutations; however, KRAS mutations were seen in 20% of tumors with BRAF mutations (72). The ErbB family consists of four receptor kinases, including ErbB1 (or EGFR) and ErbB2 [or human epidermal growth factor receptor 2 (HER2)]. Mutations in the EGFR gene were seen in 15% of CCA cases (73). MET is an oncogene that encodes for the hepatocyte growth factor (HGF) receptor. HGF/MET pathway is less established but may be important in development and progression of BTC/CCA. MET is a key regulator of invasive growth. Interaction of HGF and its receptor MET can activate many pathways including MAPK, PI3K and STAT. Overexpression of MET occurs in 12–58% of cases of iCCA and has been linked to overexpression of members of the EGFR family and shown the capacity of HGF to stimulate migration and invasion in CC cells (74).
Loss-of-function mutations in tumor suppressor genes also play a role in CCA. CDKN2A (p16INK4a) negatively regulates proliferation in normal cells and is capable of cell cycle arrest. This tumor suppressor gene was highly mutated in reports with 55% loss-of-function in iCCA and 83% in eCCA. TP53, a principal regulator of cell division, appears to be inactivated in approximately one-third of BTC, both iCCA and eCCA. SMAD4, in conjunction with the other SMAD proteins, is an end effector in the TGFβ pathway with promotes epithelial-mesenchymal transition, directly regulating the activity of genes controlling cell proliferation. Mutations in SMAD4 were described in up to 40% in iCCA with the relationship of disease staging (75). A recent large cohort study with 103 iCCA in identification of an iCCA-specific mutation signature that is associated with liver inflammation, fibrosis and cirrhosis (76). The study found that TP53-defection is more likely to be HBsAg-seropositive, whereas KRAS mutations are nearly exclusively in HBsAg-seronegative CCA patients. The study demonstrated that three pathways (Ras/PI3K, p53/cell cycle, and TGFβ/Smad), genes important for epigenetic regulations and oxidative phosphorylation are substantially affected in iCCA.
Potential role of stem and progenitor cells
Besides the different carcinogenetic mechanisms driven by risk factors in BTC, the distinct genetic profiles are also reflecting two distinct stem cell niches, the canals of Hering harboring hepatic stem cells (HpSCs) and the peribiliary glands harboring biliary tree stem/progenitor cells (BTSCs) (77). Cell populations from HpSCs and BTSCs lineages may represent distinct candidate cells of origin during CC carcinogenesis, susceptible to distinct risk factors and responsible for the development of the different iCCA and eCCA or GBC subtypes: e.g., BTSC lineage may be activated under pathological conditions affecting the large intrahepatic and extrahepatic bile ducts (including liver flukes, cholangitis, PSC, hepatolithiasis, etc.), giving rise to large bile duct pure mucin secreting iCCA and eCCA; conversely, the hHpSC lineage has been suggested to be activated in response to parenchymal liver diseases (such as chronic viral/non-viral liver disease, schistosomiasis and liver cirrhosis) and to be involved in the development of combined hepatocellular carcinoma-iCCA, bile ductular iCCA and mixed iCC A (with a focal hepatocytic differentiation, ductular reaction and mucin-secreting adenocarcinoma). This stem cell compartment is probably activated also during nonalcoholic steatohepatitis (NASH) and asbestos exposure, as these two risk factors are exclusively associated with the development of ICC.
Summary and future directions
Despite some advances in treatment of BTCs, the overall outcomes of the disease remain poor. With learning from ‘target intended’ studies and emerging understanding of the heterogeneity and the molecular landscape of BTC, future target oriented research/studies should be based on the underlining etiology, the specific genetic profile of each subgroup, and cancer-stroma microenvironment of the selected particular disease population. A recent retrospective study showed the promising therapy with blockage of Her-2/neu in BTC (with both GBC and CCA) patients who with the gene amplification (78). Advances in immunotherapy may also provide new opportunities for treating BTC (79). A complete response (CR) was reported in a chemotherapy refractory metastatic iCCA patient with mismatch-repair deficiency (dMMR) after being treated with PD-1 inhibitor (80). Most current on-going ‘target-oriented’ studies are less distinctive regarding the specificity of the characteristic mechanism of the disease (Tables 3,4). However, we are certain that the future studies will be more precisely to meet our goal.
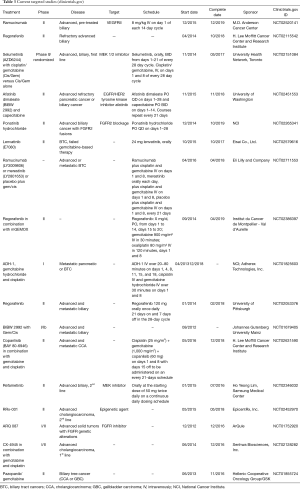
Full table

Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168-79. [Crossref] [PubMed]
- Rizvi S, Borad MJ, Patel T, et al. Cholangiocarcinoma: molecular pathways and therapeutic opportunities. Semin Liver Dis 2014;34:456-64. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Ellis PA, Norman A, Hill A, et al. Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. European Journal of Cancer 1995;31A:1594-8. [Crossref] [PubMed]
- Patt YZ, Jones DV Jr, et al. Phase II trial of intravenous flourouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol 1996;14:2311-5. [Crossref] [PubMed]
- Ducreux M, Rougier P, Fandi A, et al. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol 1998;9:653-6. [Crossref] [PubMed]
- Raderer M, Hejna MH, Valencak JB, et al. Two consecutive phase II studies of 5-fluorouracil/leucovorin/mitomycin C and of gemcitabine in patients with advanced biliary cancer. Oncology 1999;56:177-80. [Crossref] [PubMed]
- Choi CW, Choi IK, Seo JH, et al. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J Clin Oncol 2000;23:425-8. [Crossref] [PubMed]
- Patt YZ, Hassan MM, Lozano RD, et al. Phase II trial of cisplatin, interferon alpha-2b, doxorubicin, and 5-fluorouracil for biliary tract cancer. Clin Cancer Res 2001;7:3375-80. [PubMed]
- Chen JS, Lin YC, Jan YY, et al. Mitomycin C with weekly 24-h infusion of high-dose 5-fluorouracil and leucovorin in patients with biliary tract and periampullar carcinomas. Anticancer Drugs 2001;12:339-43. [Crossref] [PubMed]
- Kim TW, Chang HM, Kang HJ, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol 2003;14:1115-20. [Crossref] [PubMed]
- Kornek GV, Schuell B, Laengle F, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol 2004;15:478-83. [Crossref] [PubMed]
- Ueno H, Okusaka T, Ikeda M, et al. Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer 2004;91:1769-74. [Crossref] [PubMed]
- Rao S, Cunningham D, Hawkins RE, et al. Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br J Cancer 2005;92:1650-4. [Crossref] [PubMed]
- Cho JY, Paik YH, Chang YS, et al. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer 2005;104:2753-8. [Crossref] [PubMed]
- Cho JY, Nam JS, Park MS, et al. A Phase II study of capecitabine combined with gemcitabine in patients with advanced gallbladder carcinoma. Yonsei Med J 2005;46:526-31. [Crossref] [PubMed]
- Ducreux M, Van Cutsem E, Van Laethem JL, et al. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer 2005;41:398-403. [Crossref] [PubMed]
- Park SH, Park YH, Lee JN, et al. Phase II study of epirubicin, cisplatin, and capecitabine for advanced biliary tract adenocarcinoma. Cancer 2006;106:361-5. [Crossref] [PubMed]
- Hong YS, Lee J, Lee SC, et al. Phase II study of capecitabine and cisplatin in previously untreated advanced biliary tract cancer. Cancer Chemother Pharmacol 2007;60:321-8. [Crossref] [PubMed]
- Feisthammel J, Schoppmeyer K, Mössner J, et al. Irinotecan with 5-FU/FA in advanced biliary tract adenocarcinomas: a multicenter phase II trial. Am J Clin Oncol 2007;30:319-24. [Crossref] [PubMed]
- Furuse J, Okusaka T, Ohkawa S, et al. A phase II study of uracil-tegafur plus doxorubicin and prognostic factors in patients with unresectable biliary tract cancer. Cancer Chemother Pharmacol 2009;65:113-20. [Crossref] [PubMed]
- Penz M, Kornek GV, Raderer M, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol 2001;12:183-6. [Crossref] [PubMed]
- Gebbia V, Giuliani F, Maiello E, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol 2001;19:4089-91. [Crossref] [PubMed]
- Kuhn R, Hribaschek A, Eichelmann K, et al. Outpatient therapy with gemcitabine and docetaxel for gallbladder, biliary, and cholangio-carcinomas. Invest New Drugs 2002;20:351-6. [Crossref] [PubMed]
- Bhargava P, Jani CR, Savarese DM, et al. Gemcitabine and irinotecan in locally advanced or metastatic biliary cancer: preliminary report. Oncology (Williston Park) 2003;17:23-6. [PubMed]
- André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339-43. [Crossref] [PubMed]
- Knox JJ, Hedley D, Oza A, et al. Gemcitabine concurrent with continuous infusional 5-fluorouracil in advanced biliary cancers: a review of the Princess Margaret Hospital experience. Ann Oncol 2004;15:770-4. [Crossref] [PubMed]
- Alberts SR, Al-Khatib H, Mahoney MR, et al. Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group phase II trial. Cancer 2005;103:111-8. [Crossref] [PubMed]
- Thongprasert S, Napapan S, Charoentum C, et al. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol 2005;16:279-81. [Crossref] [PubMed]
- Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 2005;23:2332-8. [Crossref] [PubMed]
- Lee GW, Kang JH, Kim HG, et al. Combination chemotherapy with gemcitabine and cisplatin as first-line treatment for immunohistochemically proven cholangiocarcinoma. Am J Clin Oncol 2006;29:127-31. [Crossref] [PubMed]
- Kim ST, Park JO, Lee J, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 2006;106:1339-46. [Crossref] [PubMed]
- Harder J, Riecken B, Kummer O, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer 2006;95:848-52. [Crossref] [PubMed]
- Manzione L, Romano R, Germano D. Chemotherapy with gemcitabine and oxaliplatin in patients with advanced biliary tract cancer: a single-institution experience. Oncology 2007;73:311-5. [Crossref] [PubMed]
- Alberts SR, Sande JR, Foster NR, et al. Pemetrexed and gemcitabine for biliary tract and gallbladder carcinomas: a North Central Cancer Treatment Group (NCCTG) phase I and II Trial, N9943. J Gastrointest Cancer 2007;38:87-94. [Crossref] [PubMed]
- Riechelmann RP, Townsley CA, Chin SN, et al. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer 2007;110:1307-12. [Crossref] [PubMed]
- Lee J, Kim TY, Lee MA, et al. Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol 2008;61:47-52. [Crossref] [PubMed]
- André T, Reyes-Vidal JM, Fartoux L, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer 2008;99:862-7. [Crossref] [PubMed]
- Meyerhardt JA, Zhu AX, Stuart K, et al. Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci 2008;53:564-70. [Crossref] [PubMed]
- Kim HJ, Lee NS, Lee SC, et al. A phase II study of gemcitabine in combination with oxaliplatin as first-line chemotherapy in patients with inoperable biliary tract cancer. Cancer Chemother Pharmacol 2009;64:371-7. [Crossref] [PubMed]
- Jang JS, Lim HY, Hwang IG, et al. Gemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group phase II trial. Cancer Chemother Pharmacol 2010;65:641-7. [Crossref] [PubMed]
- Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896-902. [Crossref] [PubMed]
- Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol 2010;11:48-54. [Crossref] [PubMed]
- Bengala C, Bertolini F, Malavasi N, et al. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer 2010;102:68-72. [Crossref] [PubMed]
- El-Khoueiry AB, Rankin CJ, Ben-Josef E, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs 2012;30:1646-51. [Crossref] [PubMed]
- Yi JH, Thongprasert S, Lee J, et al. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur J Cancer 2012;48:196-201. [Crossref] [PubMed]
- Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 2006;24:3069-74. [Crossref] [PubMed]
- Chiorean EG, Ramasubbaiah R, Yu M, et al. Phase II trial of erlotinib and docetaxel in advanced and refractory hepatocellular and biliary cancers: Hoosier Oncology Group GI06-101. Oncologist 2012;17:13. [Crossref] [PubMed]
- Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2012;13:181-8. [Crossref] [PubMed]
- Paule B, Herelle MO, Rage E, et al. Cetuximab plus gemcitabine-oxaliplatin (GEMOX) in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology 2007;72:105-10. [Crossref] [PubMed]
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [Crossref] [PubMed]
- Jensen LH, Lindebjerg J, Ploen J, et al. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann Oncol 2012;23:2341-6. [Crossref] [PubMed]
- Sohal DP, Mykulowycz K, Uehara T, et al. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann Oncol 2013;24:3061-5. [Crossref] [PubMed]
- Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol 2009;64:777-83. [Crossref] [PubMed]
- Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 2011;29:2357-63. [Crossref] [PubMed]
- Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 2010;28:3491-7. [Crossref] [PubMed]
- El-Khoueiry AB, Rankin C, Siegel AB, et al. S0941: a phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br J Cancer 2014;110:882-7. [Crossref] [PubMed]
- Chen LT, Chen JS, Chao Y, et al. KRAS mutation status-stratified randomized phase II trial of GEMOX with and without cetuximab in advanced biliary tract cancer (ABTC): The TCOG T1210 trial. J Clin Oncol 2013;31:abstr 4018.
- Eckel F, Schmid RM. Chemotherapy and targeted therapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Chemotherapy 2014;60:13-23. [Crossref] [PubMed]
- Ross JS, Wang K, Gay L, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 2014;19:235-42. [Crossref] [PubMed]
- Simbolo M, Fassan M, Ruzzenente A, et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget 2014;5:2839-52. [Crossref] [PubMed]
- Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology 2014;59:2397-402. [Crossref] [PubMed]
- Ling H, Roux E, Hempel D, et al. Transforming growth factor β neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS One 2013;8:e54499. [Crossref] [PubMed]
- Mertens JC, Fingas CD, Christensen JD, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res 2013;73:897-907. [Crossref] [PubMed]
- Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173-84. [Crossref] [PubMed]
- Dwyer JR, Sever N, Carlson M, et al. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem 2007;282:8959-68. [Crossref] [PubMed]
- Yoon JH, Higuchi H, Werneburg NW, et al. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology 2002;122:985-93. [Crossref] [PubMed]
- Yoon JH, Canbay AE, Werneburg NW, et al. Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology 2004;39:732-8. [Crossref] [PubMed]
- Ross JS, Wang K, Catenacci DV, et al. Comprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and genomic alterations. J Clin Oncol 2015;33:abstr 231.
- Graham RP, Barr Fritcher EG, Pestova E, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol 2014;45:1630-8. [Crossref] [PubMed]
- Ong CK, Subimerb C, Pairojkul C, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet 2012;44:690-3. [Crossref] [PubMed]
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut 2003;52:706-12. [Crossref] [PubMed]
- Leone F, Cavalloni G, Pignochino Y, et al. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clin Cancer Res 2006;12:1680-5. [Crossref] [PubMed]
- Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008;7:504-16. [Crossref] [PubMed]
- Kang YK, Kim WH, Jang JJ. Expression of G1-S modulators (p53, p16, p27, cyclin D1, Rb) and Smad4/Dpc4 in intrahepatic cholangiocarcinoma. Hum Pathol 2002;33:877-83. [Crossref] [PubMed]
- Zou S, Li J, Zhou H, et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun 2014;5:5696. [Crossref] [PubMed]
- Brandi G, Farioli A, Astolfi A, et al. Genetic heterogeneity in cholangiocarcinoma: a major challenge for targeted therapies. Oncotarget 2015;6:14744-53. [Crossref] [PubMed]
- Javle M, Churi C, Kang HC, et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol 2015;8:58. [Crossref] [PubMed]
- Takahashi R, Yoshitomi M, Yutani S, et al. Current status of immunotherapy for the treatment of biliary tract cancer. Hum Vaccin Immunother 2013;9:1069-72. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]


