The role of chemotherapy in gastric cancer-related microangiopathic haemolytic anaemia
Introduction
Microangiopathic haemolytic anaemia (MAHA) and thrombocytopenia are the key clinical features of thrombotic microangiopathy (TMA) syndromes, which include primary and secondary TMA syndromes.(1) Common underlying disorders associated with secondary TMA include malignancy, systemic infection, severe hypertension and severe pre-eclampsia, eclampsia or HELLP syndrome (1). Cancer-related MAHA (CR-MAHA) refers to non-immune haemolytic anaemia caused by intravascular red blood cell fragmentation in the setting of malignancy and was first described by Brain et al. (2). Investigations typically reveal a negative Coombs’ test and fragmented red blood cells, or schistocytes, on peripheral blood smear. Gastric cancer is the most commonly reported malignancy associated with MAHA, followed by breast, prostate, lung and cancer of unknown origin (3). We report a case of a patient presenting with CR-MAHA secondary to a gastric adenocarcinoma treated with plasmapheresis and chemotherapy. We also review previous cases of gastric cancer-associated MAHA and current understanding of their pathophysiology and optimal treatment.
Case presentation
A previously well 46-year-old man presented with several weeks of increasing lethargy and 2 days of palpitations and shortness of breath on exertion. The patient denied any episodes of melaena, rectal bleeding or haematemesis. He did not have any significant medical or family history, did not take regular medications and had no known drug allergies.
On examination, the patient appeared pale and was neurologically intact. His blood pressure was stable, but he was febrile to 38.1 °C and mildly tachycardic with a heart rate of 104 beats per minute. His abdomen was soft and non-tender, with a palpable liver edge and tippable spleen.
A full blood count revealed normocytic, normochromic anaemia with a haemoglobin of 53 g/L. He was thrombocytopenic with platelets of 82×109/L and his white cells were mildly elevated at 11.36×109/L. Haematinic studies showed normal ferritin, iron saturation and red cell folate levels. A positive haemolysis screen revealed elevated LDH (553 unit/L), elevated reticulocytes (223.6×109/L) and low haptoglobin (<0.1 g/L). A direct antiglobulin test (Direct Coombs test) was negative. His blood film showed marked polychromasia, spherocytes, schistocytes and nucleated red blood cells consistent with MAHA. Coagulation studies showed mildly elevated PT 18.7 and INR 1.6, with normal APTT 29.6. His ADAMTS13 levels were normal at 30%, as was his creatinine at 89 µmol/L.
The patient was admitted for further inpatient investigation of his thrombocytopenia and anaemia. Early in his admission, he was transfusion-dependent and required up to 2 units of packed red blood cells each day. He also received multiple platelet transfusions when his platelets fell to <20×109/L. Various investigations performed to look for an underlying cause of his abnormal blood count were normal, including HIV antigen/antibody testing, serum electrophoresis and autoimmune screen (ANA, ANCA, dsDNA, C3 and C4 levels, ENA).
A Vascath was inserted to facilitate treatment with 7 days of plasmapheresis in conjunction with prednisone 100 mg daily, with no resultant improvement in the patient’s anaemia. A bone marrow aspirate and trephine showed a hypercellular bone marrow with infiltration by non-haemotopoietic cells. On immunohistochemistry, these infiltrating cells were consistent with adenocarcinoma, likely arising from the upper gastro-intestinal tract.
Gastroscopy revealed an actively bleeding, ulcerated, malignant gastric tumour on the anterior wall and greater curvature of the stomach. Computed tomography (CT) of his abdomen and chest showed widespread non-specific prominent upper abdominal para-aortic lymph nodes measuring up to 10 mm with no evidence of any visceral lesions. A FDG-PET scan showed tracer uptake in his primary gastric tumour, as well as in loco-regional abdominal lymph nodes, axial skeleton and medullary cavities of long bones consistent with bone metastases and bone marrow infiltration.
The patient was commenced on modified FOLFOX-6 chemotherapy (5-fluorouracil 2,400 mg/m2 continuous intravenous infusion over 46 h D1, leucovorin 350 mg D1, oxaliplatin 85 mg/m2 D1 q2weekly) for metastatic gastric cancer. After 2 cycles of chemotherapy, his haemoglobin stabilised at 91 g/L. He did not require further red blood cell transfusions and his haemoglobin continued to rise to 125 g/L with further cycles of chemotherapy (Figure 1). The patient’s CA19.9 was elevated at 332 kU/L when measured shortly after commencing chemotherapy and fell with further cycles of treatment. Progress CT scan showed a decrease in the size of his intra-abdominal lymphadenopathy, and gastric thickening and sclerotic bone metastases which have remained stable over a 7-month period.
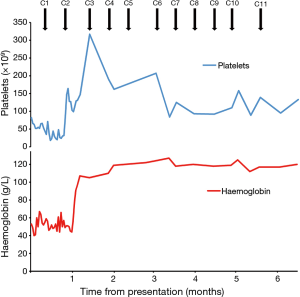
The patient was initially treated with 11 cycles of modified FOLFOX-6 chemotherapy. His oxaliplatin was subsequently ceased due to tetany and early neuropathic symptoms. He continues on treatment with single agent 5FU (5-fluorouracil 2,400 mg/m2 continuous intravenous infusion over 46 h D1, leucovorin 350 mg D1, q2weekly) and remains transfusion-free more than 7 months after his initial presentation.
Discussion
We identified 24 case reports of patients with gastric cancer-associated MAHA who underwent treatment with chemotherapy (Table 1) (4-24). The median age of reported cases was 51 years (range, 19–71 years) and cases were reported more frequently in men than women. This is younger than the median age at diagnosis (69 years) for the general gastric cancer population, while the gender distribution is concordant (25). Eight patients had been previously diagnosed with and treated for gastric cancer, so their haematological derangements heralded disease recurrence. Only two patients were reported to have neurological symptoms (including confusion, disorientation and seizure) while none of the cases reported associated renal dysfunction (9,13). This contrasts with neurological and renal involvement typically associated with primary TMA syndromes such as ADAMTS13 deficiency-mediated TMA and Shiga toxin-mediated TMA [previously known as thrombocytopenic purpura (TTP) and haemolytic-uraemic syndrome (HUS), respectively] (1).
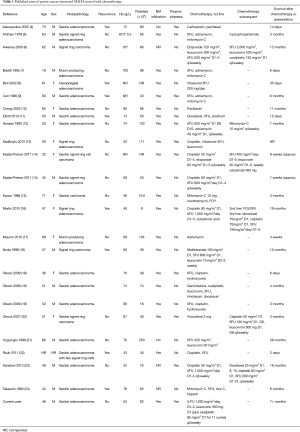
Full table
Twenty-one patients in our literature review underwent bone marrow biopsy, of whom 19 were found to have bone marrow infiltration by malignant cells. This suggests that bone marrow failure due to malignant replacement may contribute to the anaemia and thrombocytopenia seen in these patients. This is unlikely to be the only explanation for these abnormalities, however, as not all patients with malignant bone marrow infiltration develop peripheral blood features of MAHA (3). The pathogenesis of CR-MAHA is poorly understood, as it appears to differ from primary TMA syndromes such as hereditary and immune-mediated TTP and HUS (3,23). Current theories implicate red cell fragmentation and platelet destruction in small vessels of malignant tissue, potentially mediated by tumour-induced cytokine production and/or endothelial injury (3). We hypothesise that chemotherapy leads to improvement of haematological parameters by two mechanisms: it reverses the processes leading to microangiopathic destruction of red blood cells and platelets; and it also clears malignant cells from the bone marrow, allowing recovery of normal haematopoietic cells and blood counts.
In the literature, seven patients with gastric cancer-related MAHA underwent plasmapheresis, of whom only one patient experienced an improvement in clinical symptoms and haematological indices. Primary ADAMTS13 deficiency-mediated TMA is an autoimmune disorder caused by inhibition of ADAMTS13 activity by ADAMTS13-directed antibodies, which are removed during treatment with plasmapheresis (1). A case series of 4 patients with gastric cancer-associated MAHA reported that none had sufficiently low ADAMTS13 levels warranting measurement of ADAMTS13 antibodies (19). In a report of 10 patients referred for plasmapheresis for various malignancy-related MAHA, which included one case of gastric cancer, no patient had severe ADAMTS13 deficiency or responded to plasmapheresis (11). The normal ADAMTS13 levels measured in our patient is consistent with these other reports of CR-MAHA, as is his lack of improvement with plasmapheresis, providing evidence that antibodies are not implicated in the pathogenesis of CR-MAHA. Hence plasmapheresis in CR-MAHA appears to be of little use and treatment of the underlying cancer should be the focus of management.
The patients in our review were treated with a variety of chemotherapy regimens, resulting in a wide spread of survival times ranging from 6 days up to 26 months (median 3 months). Most patients (20 of 24) were treated with a fluoropyrimidine and/or platinum agent, reflecting the chemotherapy drugs in common usage in gastric carcinoma. There was no obvious association between any particular drug and prolonged survival.
In conclusion, malignancy-associated MAHA and thrombocytopenia are secondary TMA syndromes that have been reported most commonly in gastric cancers. They may represent the first presentation of metastatic gastric cancer or disease recurrence. Plasmapheresis appears to have a limited role in their treatment. This is most likely due to the absence of ADAMTS13-directed antibodies, which feature prominently in primary autoimmune TMA syndromes. Chemotherapy should be the mainstay of treatment for CR-MAHA as it improves haematological parameters, although overall prognosis is still poor with a median survival of 3 months with chemotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this article and any accompanying images.
References
- George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med 2014;371:654-66. [Crossref] [PubMed]
- Brain MC, Dacie JV, Hourihane DO. Microangiopathic haemolytic anaemia: the possible role of vascular lesions in pathogenesis. Br J Haematol 1962;8:358-74. [Crossref] [PubMed]
- Lechner K, Obermeier HL. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore) 2012;91:195-205. [Crossref] [PubMed]
- Alexopoulou A, Dourakis SP, Nomikou E. Case of thrombotic thrombocytopenic purpura associated with disseminated gastric cancer. Am J Clin Oncol 2002;25:632. [Crossref] [PubMed]
- Antman KH, Skarin AT, Mayer RJ, et al. Microangiopathic hemolytic anemia and cancer: a review. Medicine (Baltimore) 1979;58:377-84. [Crossref] [PubMed]
- Arkenau HT, Müssig O, Buhr T, et al. Microangiopathic hemolytic anemia (MAHA) as paraneoplastic syndrome in metastasized signet ring cell carcinomas: case reports and review of the literature. Z Gastroenterol 2005;43:719-22. [Crossref] [PubMed]
- Bisetti A, Lalicata M, Jacot-des-Combes E. Occult gastric adenocarcinoma with pulmonary carcinomatous lymphangitis and microangiopathic hemolytic anemia in a young adult. Schweiz Med Wochenschr 1985;115:561-4. [PubMed]
- Blot E, Decaudin D, Veyradier A, et al. Cancer-related thrombotic microangiopathy secondary to Von Willebrand factor-cleaving protease deficiency. Thromb Res 2002;106:127-30. [Crossref] [PubMed]
- Carr DJ, Kramer BS, Dragonetti DE. Thrombotic thrombocytopenic purpura associated with metastatic gastric adenocarcinoma: successful management with plasmapheresis. South Med J 1986;79:476-9. [Crossref] [PubMed]
- Chang JC, Naqvi T. Thrombotic thrombocytopenic purpura associated with bone marrow metastasis and secondary myelofibrosis in cancer. Oncologist 2003;8:375-80. [Crossref] [PubMed]
- Elliott MA, Letendre L, Gastineau DA, et al. Cancer-associated microangiopathic hemolytic anemia with thrombocytopenia: an important diagnostic consideration. Eur J Haematol 2010;85:43-50. [PubMed]
- Hansen RM, Hanson GA. Gastric carcinoma in young people. A case report and review of the literature. Am J Gastroenterol 1980;74:497-503. [PubMed]
- Kadikoylu G, Barutca S, Tataroglu C, et al. Thrombotic thrombocytopenic purpura as the first manifestation of metastatic adenocarcinoma in a young woman. Transfus Apher Sci 2010;42:39-42. [Crossref] [PubMed]
- Kaidar-Person O, Nasrallah H, Haim N, et al. Disseminated carcinoma diagnosed by bone marrow biopsy in patients with microangiopathic hemolytic anemia and thrombocytopenia: a report of two cases with gastric cancer and a review of the literature. J Gastrointest Cancer 2011;42:123-6. [Crossref] [PubMed]
- Kanou T, Nosou Y, Yoshinaka K, et al. Microangiopathic hemolytic anemia associated with gastric cancer. Gan No Rinsho 1986;32:1029-34. [PubMed]
- Martín AJ, Alfonso PG, Martínez MC, et al. Microangiopathic hemolytic anemia and diffuse bone metastasis by signet ring cell adenocarcinoma. Journal of Cancer Therapy 2010;1:94. [Crossref]
- Mauron H, Streuli R. Anemia, subcutaneous bleeding and weight loss. Disseminated metastasizing, mucinous adenocarcinoma of the stomach. Praxis (Bern 1994) 2000;89:1568-72.
- Noda N, Sano T, Shirao K, et al. A case of bone marrow recurrence from gastric carcinoma after a nine-year disease-free interval. Jpn J Clin Oncol 1996;26:472-5. [Crossref] [PubMed]
- Oberic L, Buffet M, Schwarzinger M, et al. Cancer awareness in atypical thrombotic microangiopathies. Oncologist 2009;14:769-79. [Crossref] [PubMed]
- Otrock ZK, Taher AT, Makarem JA, et al. Thrombotic thrombocytopenic purpura and bone marrow necrosis associated with disseminated gastric cancer. Dig Dis Sci 2007;52:1589-91. [Crossref] [PubMed]
- Ozgüroglu M, Demirelli F, Mandel NM. Microangiopathic hemolytic anemia as an early predictor of recurrence in gastric cancer. Am J Clin Oncol 1999;22:214. [Crossref] [PubMed]
- Rauh MJ, Al Habeeb A, Chang H. Microangiopathic hemolytic anemia and leukoerythroblastic blood film heralding bone marrow metastatic gastroesophageal adenocarcinoma. Pathol Res Pract 2011;207:121-3. [Crossref] [PubMed]
- Sanatani MS. Lazo-Langner A2, Al-Rasheedy IM3. Cisplatin and short-term 5-Fluorouracil infusion for paraneoplastic microangiopathic hemolytic anemia in gastric cancer: a case report and review of the literature. Case Rep Oncol Med 2013;2013:594787. [Crossref] [PubMed]
- Takeuchi R, Kuto M, Katayama N, et al. Carcinomatosis associated with microangiopathic hemolytic anemia and disseminated intravascular coagulation: 12 years after gastrectomy for gastric adenocarcinoma. Rinsho Ketsueki 1983;24:1423-9. [PubMed]
- Howlader NA, Krapcho M, Miller D, et al. editors. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; 2016.


